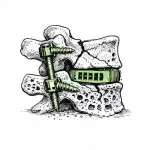
For years, spine surgery has been performed under the assumption of adequate bone quality: stiffer implants, larger corrections, more instrumented levels, and increasing use of MIS in older patients. But demographics changed before surgical philosophy did. Today, the typical degenerative case is less often a middle-aged disc herniation and increasingly an elderly patient. … [Read more...] about Most Spine Failures Are Predictable — The Bone Problem in Spine Surgery Nobody Budgets For
2026
Vivex Announces New Peer-Reviewed Publication Demonstrating Long-Term Fusion Outcomes with VIA Form+™ in Lumbar Interbody Fusion
MIAMI, Feb. 12, 2026 /PRNewswire/ -- Vivex Biologics, Inc., a leading medical technology company developing and delivering innovative allografts for musculoskeletal and wound-care applications, today announced the publication of a new peer-reviewed study evaluating the long-term clinical and radiographic outcomes of lumbar spinal fusion procedures utilizing VIA … [Read more...] about Vivex Announces New Peer-Reviewed Publication Demonstrating Long-Term Fusion Outcomes with VIA Form+™ in Lumbar Interbody Fusion
VISIE Achieves Commercial Milestone With Launch of Partner APIs
AUSTIN, Texas--(BUSINESS WIRE)--VISIE Inc. today announced the availability of its partner application programming interfaces (APIs), marking a significant milestone in the company’s commercial and integration readiness. The APIs enable surgical robotics and navigation partners to integrate VISIE’s spatial computing and real-time scanning capabilities into existing robotic … [Read more...] about VISIE Achieves Commercial Milestone With Launch of Partner APIs
We are proud to announce that Centinel Spine will once again be a sponsor of SPINEMarketGroup in 2026
Thank you, Centinel Spine! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About Centinel Spine, LLC Centinel Spine®, LLC is the leading global medical device company addressing cervical and lumbar spinal disease with the most … [Read more...] about We are proud to announce that Centinel Spine will once again be a sponsor of SPINEMarketGroup in 2026
Zavation Medical Products Unveils Next-Generation Cervical Interbody Featuring the Combined Power of NanoPrime® and Labyrinth® Porous PEEK
FLOWOOD, Miss., Feb. 11, 2026 /PRNewswire/ -- Zavation Medical Products, LLC ("Zavation"), announces the release of its most advanced cervical interbody implant to date—the integration of NanoPrime® Titanium Ion Bond Technology with the company's proprietary Labyrinth® porous PEEK architecture. This combination is engineered to elevate the mechanical advantages of PEEK with the … [Read more...] about Zavation Medical Products Unveils Next-Generation Cervical Interbody Featuring the Combined Power of NanoPrime® and Labyrinth® Porous PEEK
Spark Spine and Invictos Orthopedics Announce Strategic Partnership
Collaboration promises to let EMP technology "off the leash" in veterinary medicine. Partnering with Invictos Orthopedics allows us to apply our EMP expertise to a field that is deeply deserving of the best technologies.” — Kevin Chappuis, President of Spark SpineBOSTON, MA, UNITED STATES, February 10, 2026 /EINPresswire.com/ -- Spark Spine, a leader in advanced … [Read more...] about Spark Spine and Invictos Orthopedics Announce Strategic Partnership
Carlsmed Announces First aprevo® Bi-lateral Posterior Lumbar Interbody Fusion Procedure
CARLSBAD, Calif., Feb. 10, 2026 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), today announced the successful completion of the first posterior lumbar spine surgery using the Company’s aprevo® Lumbar Bi-lateral Posterior System. The procedure was performed by Orthopedic Spine Surgeon CJ Kleck, M.D. at the University of Colorado Hospital in … [Read more...] about Carlsmed Announces First aprevo® Bi-lateral Posterior Lumbar Interbody Fusion Procedure
NGMedical announces first implantation of Move-P for lumbar motion preservation
NGMedical GmbH is excited to announce the first in human implantation of its next generation lumbar motion preserving system MOVE®-P NONNWEILER, GERMANY, February 9, 2026 /EINPresswire.com/ -- In January, the first MOVE®-P implantation was performed in Hamburg by Dr. Ardeshir Ardeshiri. MOVE®-P is a pedicle screw based physiological motion preserving device to top-off a … [Read more...] about NGMedical announces first implantation of Move-P for lumbar motion preservation
Expandable cages were adopted not because they fused better, but because they made fusion easier to perform. Review of 64 TLIF/PLIF Expandable Systems (2026 Update)
In less than a decade, expandable interbody cages have evolved from a niche concept into one of the fastest-growing segments in lumbar fusion. The global interbody cage market is estimated at roughly $3–3.5 billion annually, with expandable designs already representing a substantial portion of posterior lumbar procedures — corresponding to an estimated $700 million to $1.1 … [Read more...] about Expandable cages were adopted not because they fused better, but because they made fusion easier to perform. Review of 64 TLIF/PLIF Expandable Systems (2026 Update)
Genesys Spine Announces Positive Prospective Study Results for SIros® Lateral SI Joint Fusion System
AUSTIN, Texas, Feb. 6, 2026 /PRNewswire/ -- Genesys Spine today announced the results of a prospective multicenter clinical study evaluating the safety and effectiveness of the SIros® Lateral Sacroiliac Joint Fusion System. The findings demonstrate significant pain reduction, functional improvement, and radiographic evidence of successful fusion, reinforcing … [Read more...] about Genesys Spine Announces Positive Prospective Study Results for SIros® Lateral SI Joint Fusion System
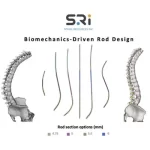
First-of-its-kind Universal Clearance Establishes Maximum Regulatory Access to the Bezier Parametric Curve Spinal Rod System for Patients and Surgeons
FORT LAUDERDALE, Fla., Feb. 5, 2026 /PRNewswire/ -- The Bezier Parametric Curve Rod System from Spinal Resources, Inc. has received 510(k) clearance for compatibility with any cleared pedicle screw set available on the US market, regardless of manufacturer. From a patient access perspective, the significance of this clearance cannot be overstated. By … [Read more...] about First-of-its-kind Universal Clearance Establishes Maximum Regulatory Access to the Bezier Parametric Curve Spinal Rod System for Patients and Surgeons
Expandable Cervical Cages: Innovation or Just a Niche Tool?
Anterior cervical discectomy and fusion (ACDF) is one of the most standardized procedures in spine surgery. The workflow is familiar: decompression, controlled distraction, sizing, and insertion of a static interbody cage. For decades, this has worked reliably and predictably. So when expandable cages appeared in the cervical spine, the reaction was immediate and … [Read more...] about Expandable Cervical Cages: Innovation or Just a Niche Tool?
We are proud to announce that iSpine will be a Sponsor of SPINEMarketGroup in 2026!
Thank you, iSpine! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About iSpine iSpine is a South African company that was formed to meet the needs of Neuro and Spinal surgeons the world over.iSpine and its expert partners, designs, … [Read more...] about We are proud to announce that iSpine will be a Sponsor of SPINEMarketGroup in 2026!
Providence Medical Technology Appoints Industry Veteran Jim Hens as Chief Commercial Officer as the Company Continues Rapid Growth Following Publication of Landmark Study
PLEASANTON, Calif., Feb. 4, 2026 /PRNewswire/ -- Providence Medical Technology (Providence), a pioneer in spinal fusion surgery technology, today announced the appointment of industry veteran Jim Hens as Chief Commercial Officer. Hens has over 25 years of leadership experience in the orthopedic and spine industries and is joining the company to spearhead its … [Read more...] about Providence Medical Technology Appoints Industry Veteran Jim Hens as Chief Commercial Officer as the Company Continues Rapid Growth Following Publication of Landmark Study
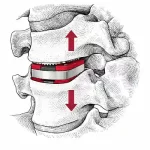
Why Expandable Cages Sometimes Lose Lordosis After Lumbar Fusion
Expandable interbody cages have earned their place in modern lumbar fusion—not because they’re trendy, but because they’ve genuinely changed what surgeons can accomplish. The ability to restore disc height and segmental lordosis through controlled, in‑situ expansion has reshaped surgical strategy in meaningful ways. Add to that the smaller insertion profiles, reduced endplate … [Read more...] about Why Expandable Cages Sometimes Lose Lordosis After Lumbar Fusion
We are proud to announce that LfC will be a Sponsor of SPINEMarketGroup in 2026!
Thank you, LfC! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About LfC LfC is a polish company who have achieved a leading position in the design and manufacture of surgical equipment used in spinal treatment in orthopaedics and … [Read more...] about We are proud to announce that LfC will be a Sponsor of SPINEMarketGroup in 2026!
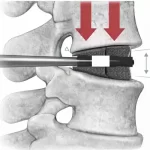
Medical Device Correction Addresses Loss of Lordosis Risk in Expandable Interbody Cages
In September 2025, Medtronic initiated an Urgent Medical Device Correction related to its Catalyft™ PL and PL40 expandable interbody systems, aimed at mitigating the risk of post-implantation loss of lordosis, also described as loss of height or cage collapse. The correction was initiated on September 16, 2025, and posted publicly on November 21, 2025, following internal … [Read more...] about Medical Device Correction Addresses Loss of Lordosis Risk in Expandable Interbody Cages
CBYON Surpasses 4,500 Navigated Surgical Cases Across Spine, Cranial, and ENT Procedures
This Milestone reflects real-world adoption of CBYON’s navigation platform and standardized operating room execution across hospitals nationwide. This milestone represents trust built over time” — Brian Moore TEMPE, AZ, UNITED STATES, February 2, 2026 /EINPresswire.com/ -- CBYON today announced a significant clinical milestone, surpassing 4,500 navigated surgical cases … [Read more...] about CBYON Surpasses 4,500 Navigated Surgical Cases Across Spine, Cranial, and ENT Procedures
We are proud to announce that NGMedical will be a Sponsor of SPINEMarketGroup in 2026!
Thank you, NGMedical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About NGMedical NGMedical combines science and innovation and stands for the development and production of highly innovative implants for spine surgery. … [Read more...] about We are proud to announce that NGMedical will be a Sponsor of SPINEMarketGroup in 2026!