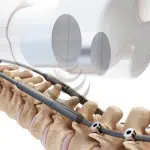
Mediplast AB is delighted to announce its new strategic partnership with icotec, a global leader in Carbon/PEEK spinal implant technologies. This collaboration will expand Mediplast’s orthopedic and spinal surgery portfolio, introducing the BlackArmor® radiolucent implant solutions to Nordic markets, specifically designed for spine tumor surgery. These state-of-the-art implants … [Read more...] about Invisibly Better – Now Together with Mediplast: A Partnership to Revolutionize Spine Surgery in Oncology
2025
Highridge Moves Fast, Acquires Key Spine Assets – Why This Move? Aiming for Market Leadership?
The spinal surgery device market is undergoing an unprecedented transformation. In recent years, several leading companies have executed strategic moves that have reshaped the competitive landscape. Globus Medical acquired NuVasive, consolidating its presence in minimally invasive spine solutions; Stryker decided to sell its Spine division to Viscogliosi Brothers, while Zimmer … [Read more...] about Highridge Moves Fast, Acquires Key Spine Assets – Why This Move? Aiming for Market Leadership?
Highridge Medical Acquires Innovative Products from Accelus to Expand Capabilities in Spine Surgery
WESTMINSTER, Colo., Sept. 02, 2025 (GLOBE NEWSWIRE) -- Highridge Medical, one of the world’s largest privately held spine companies with an innovative portfolio supported by extensive clinical evidence, announced today its acquisition of the FlareHawk® and Toro™ expandable interbody fusion systems, as well as the LineSider® pedicle screw system from Accelus, a leader … [Read more...] about Highridge Medical Acquires Innovative Products from Accelus to Expand Capabilities in Spine Surgery
Intrinsic Therapeutics Announces the First Implantation of the Barricaid® Annular Closure Device Under Awake Sedation
BOSTON, Sept. 2, 2025 /PRNewswire/ -- Intrinsic Therapeutics, Inc., a medical technology company committed to redefining the standard of care for lumbar discectomy patients with large annular defects by reducing reoperations for reherniations by 81%* with its Barricaid Annular Closure Device, announced today that, for the first time, … [Read more...] about Intrinsic Therapeutics Announces the First Implantation of the Barricaid® Annular Closure Device Under Awake Sedation
UPDATE – Carlsmed® Reports Second Quarter 2025 Financial Results
CARLSBAD, Calif., Aug. 28, 2025 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), a medical technology company pioneering AI-enabled personalized spine surgery solutions, today reported financial results for the second quarter ended June 30, 2025. “Our strong commercial performance was driven by the continued adoption of our highly … [Read more...] about UPDATE – Carlsmed® Reports Second Quarter 2025 Financial Results
Managing Early-Onset Scoliosis: Which Growth-Preserving Treatments Are Clinically Approved? Exploring Innovations and the Future of EOS Management.-Updated September 2025
Corrigendum: This article has been updated to reflect that MARVEL™ and REFLECT™ are FDA-approved devices, correcting previous descriptions that referred to them as investigational. Author’s Note:Early-onset scoliosis (EOS) remains a complex condition, and research continues to explore more effective ways of managing it. With this article, my goal was to provide an overview … [Read more...] about Managing Early-Onset Scoliosis: Which Growth-Preserving Treatments Are Clinically Approved? Exploring Innovations and the Future of EOS Management.-Updated September 2025
Globus, Life Spine, and the Patent Battleground: Who Sets the Rules? Is the Courtroom the New Operating Room for Spine Innovation?
On August 28, 2025, a Delaware jury ruled that Life Spine’s ProLift expandable interbody implants infringed multiple claims of a valid Globus Medical patent tied to its RISE® expandable cage platform. The panel awarded Globus roughly $9.5 million in damages — about $6 million in lost profits and $3.5 million in reasonable royalties, according to court filings and company … [Read more...] about Globus, Life Spine, and the Patent Battleground: Who Sets the Rules? Is the Courtroom the New Operating Room for Spine Innovation?
Globus Medical, Inc. Secures Jury Verdict in Delaware Patent Litigation Against Life Spine, Inc.
AUDUBON, Pa., Aug. 28, 2025 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal technology company, announced that a Delaware jury has returned a verdict in favor of Globus in its patent infringement litigation against Life Spine, Inc. The jury found that Life Spine’s Prolift family of implants … [Read more...] about Globus Medical, Inc. Secures Jury Verdict in Delaware Patent Litigation Against Life Spine, Inc.
Biedermann Medtech Group Announces Transformation of U.S. Spine Market Approach and New Business Model
MIAMI--(BUSINESS WIRE)--The Biedermann Medtech Group, the prominent innovator in next generation spinal and extremity implant systems and procedural solutions, today announced a strategic transformation of its U.S. operations. Biedermann will expand its focus beyond product distribution to a broader platform of integrated services and solutions designed to reshape its U.S. … [Read more...] about Biedermann Medtech Group Announces Transformation of U.S. Spine Market Approach and New Business Model
PainTEQ Secures Majority Growth Recapitalization Co-Led by Signet Healthcare Partners & Windham Capital Partners
TAMPA, Fla., Aug. 27, 2025 /PRNewswire/ -- PainTEQ, a leader in minimally invasive solutions for sacroiliac (SI) joint dysfunction, today announced a majority growth recapitalization co-led by Signet Healthcare Partners and Windham Capital Partners, two U.S.-based healthcare investment firms with nearly 50 years of combined investment experience in medical technology … [Read more...] about PainTEQ Secures Majority Growth Recapitalization Co-Led by Signet Healthcare Partners & Windham Capital Partners
Intech, Tyber Medical, and Resolve Surgical Technologies Form Two High-Impact Business Units and Appoint New Leadership
BETHLEHEM, PA, UNITED STATES, August 27, 2025 /EINPresswire.com/ -- In a bold move to elevate performance and accelerate innovation across the MedTech industry, the combined organization of Intech, Tyber Medical, and Resolve Surgical Technologies today announced a strategic reorganization into two distinct operational business units: • Manufacturing Solutions (‘MS BU’) – … [Read more...] about Intech, Tyber Medical, and Resolve Surgical Technologies Form Two High-Impact Business Units and Appoint New Leadership
Driving Force Acquires Vertebral Motion Analysis (VMA®) Technology from Wenzel Spine
CHICAGO--(BUSINESS WIRE)--Driving Force today announced the acquisition of the groundbreaking Vertebral Motion Analysis (VMA®) technology from Wenzel Spine, marking a pivotal step in the advancement of spinal imaging and diagnostics. The acquisition positions Driving Force VMA as the sole owner and operator of the most advanced, FDA-cleared motion imaging system for diagnosing … [Read more...] about Driving Force Acquires Vertebral Motion Analysis (VMA®) Technology from Wenzel Spine
Curiteva Launches the Inspire Anterior Lumbar Portfolio
HUNTSVILLE, Ala., August 26, 2025 (Newswire.com) - Curiteva, Inc., a leading technology and manufacturing company, proudly announces the release of the first offering from its innovative Anterior Lumbar Inspire Portfolio. This advanced platform includes implant offerings in both monolithic and stand-alone designs, complemented by a comprehensive plating system for … [Read more...] about Curiteva Launches the Inspire Anterior Lumbar Portfolio
VADER® Pedicle System – CE Approval under MDR for Line Extension & Navigated Instruments
August 22, 2025 – Altstätten, Switzerland – icotec ag, the leading medical device manufacturer of Carbon/PEEK spinal implants, announces the CE approval under the European Medical Device Regulation (MDR) to market the VADER® Pedicle System inclusive of Ø 4.5 mm pedicle screws and long Carbon/PEEK rods made from BlackArmor® and its use with Medtronic StealthStation™ navigation … [Read more...] about VADER® Pedicle System – CE Approval under MDR for Line Extension & Navigated Instruments
Medtronic announces Board appointments and shareholder value creation initiatives to advance strategic priorities
GALWAY, Ireland, Aug. 19, 2025 /PRNewswire -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced the Medtronic Board of Directors has appointed John Groetelaars and Bill Jellison as independent directors, effective immediately. Consistent with the company's commitment to shareholder value creation, and to provide enhanced focus and urgency … [Read more...] about Medtronic announces Board appointments and shareholder value creation initiatives to advance strategic priorities
Spine Market Leadership: Globus Pressures Medtronic at the Finish Line!
Medtronic has long dominated the spine market, leading the way with its global reach and extensive portfolio. But recent results suggest that its leadership is no longer assured. Globus Medical, a smaller yet fast-growing rival, has emerged as an aggressive and ambitious competitor—especially following its acquisition of NuVasive—and is now clearly aiming to challenge … [Read more...] about Spine Market Leadership: Globus Pressures Medtronic at the Finish Line!
Medtronic reports first quarter fiscal 2026 financial results
GALWAY, Ireland, Aug. 19, 2025 /PRNewswire -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for its first quarter (Q1) of fiscal year 2026 (FY26), which ended July 25, 2025. Key Highlights "We delivered another consistent quarter of mid-single digit organic revenue growth, with broad strength from several … [Read more...] about Medtronic reports first quarter fiscal 2026 financial results
Eminent Spine Reports 70% Growth in Trailing 12-Month Income and Celebrates Alpha Launch of FDA-Approved 3D Printed Titanium Pedicle Screw
Plano, TX, August 18, 2025 --(PR.com)-- Eminent Spine announced today that its trailing twelve-month total income for the period ending June 2025 has risen by nearly 70%, underscoring the company’s strong momentum and expanding presence in the spine market. This milestone comes as the company celebrates the Alpha Launch of the world’s only FDA-approved 3D Printed Titanium … [Read more...] about Eminent Spine Reports 70% Growth in Trailing 12-Month Income and Celebrates Alpha Launch of FDA-Approved 3D Printed Titanium Pedicle Screw
Intrinsic Therapeutics Appoints Industry Veterans Mike Lytle and Dave Decker to Lead Sales and Advance Discectomy Treatment
BOSTON, Aug. 18, 2025 /PRNewswire/ -- Intrinsic Therapeutics, Inc., a medical technology company committed to redefining the standard of care for lumbar discectomy patients with large annular defects by reducing reoperations for reherniations by 81%* with its Barricaid Annular Closure Device, announced today the appointment of industry veterans Mike Lytle and Dave Decker as … [Read more...] about Intrinsic Therapeutics Appoints Industry Veterans Mike Lytle and Dave Decker to Lead Sales and Advance Discectomy Treatment