Today, I would like to share the following reflection with all of you: Artificial intelligence (AI) is a topic that is everywhere today. There is almost no subject or conversation in which it does not appear, and in which we do not talk about its impact on the future. But, as often happens, there is a lot of discussion about what will happen in the future, while we barely see … [Read more...] about Some Thoughts and Data on AI in the Spine Market Today: What Now Seems Like a Distant Future Is Already Being Built?
NEWS
EXALTA Group Receives FDA 510(k) Clearance for New Anterior Cervical Plating System
MARQUETTE, MI, UNITED STATES, November 13, 2025 /EINPresswire.com/ -- EXALTA Group, a next-generation partner to MedTech innovators, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Anterior Cervical Plating (ACP) System, designed to support stability and fusion in the cervical spine. The ACP system is indicated for … [Read more...] about EXALTA Group Receives FDA 510(k) Clearance for New Anterior Cervical Plating System
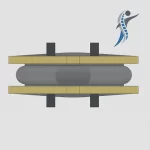
Spinvention: An Emerging Innovator in Motion-Preserving Spinal Care to Watch
Spinvention is one of those innovators worth paying close attention to. They have developed a motion-preserving spinal implant protected by a broad intellectual property portfolio across the United States, Europe (Unitary Patent), Japan, and additional international markets. Its design is based on a dual-core architecture that closely replicates the biomechanics of a natural … [Read more...] about Spinvention: An Emerging Innovator in Motion-Preserving Spinal Care to Watch
SpineGuard obtains a U.S. patent on its universal adapter for orthopaedic power drills and robots
PARIS and BOULDER (CO), November 24, 2025 – 6:00 pm CET - SpineGuard (FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced that its patent application directed to protect a universal adapter for securing bone drilling power … [Read more...] about SpineGuard obtains a U.S. patent on its universal adapter for orthopaedic power drills and robots
2025 Update: Posterior Cervical Spine Systems Market – 70 Products: Current Landscape
The global posterior cervical spine system market was valued at approximately USD 2,000 million in 2024. It is expected to grow to around USD 3,800 million by 2035, representing a compound annual growth rate (CAGR) of about 6.0% from 2025 to 2035 (WiseGuy Reports, 2024). A more specific report focusing on posterior cervical fixation and reconstruction systems estimates that … [Read more...] about 2025 Update: Posterior Cervical Spine Systems Market – 70 Products: Current Landscape
SMAIO Appoints Matthieu Ville as Chief Commercial Officer to Accelerate Commercialization and Strengthen Its Leadership in Spinal Realignment
Matthieu Ville brings over 15 years of experience in medical marketing in the United States. DALLAS & LYON, France--(BUSINESS WIRE)--Regulatory News:SMAIO (Software, Machines and Adaptative Implants in Orthopaedics– Euronext Growth Paris, ISIN: FR0014005I80 / Ticker: ALSMA), a French-American company specialized in complex spine surgery with a global offer comprising … [Read more...] about SMAIO Appoints Matthieu Ville as Chief Commercial Officer to Accelerate Commercialization and Strengthen Its Leadership in Spinal Realignment
Court Filing Update: Gizmo Medical Founder and Former Life Spine CEO Vindicated After Court Dismisses Majority of Life Spine’s Claims and Company Withdraws Remaining Allegations
CHICAGO--(BUSINESS WIRE)--Gizmo Medical announces that Life Spine has voluntarily dismissed its lawsuit against founder and former CEO Michael Butler, following a court ruling that dismissed the majority of the company’s claims before discovery even commenced. The now-dismissed Life Spine lawsuit was commenced several months after Mr. Butler filed a lawsuit in the Cook … [Read more...] about Court Filing Update: Gizmo Medical Founder and Former Life Spine CEO Vindicated After Court Dismisses Majority of Life Spine’s Claims and Company Withdraws Remaining Allegations
Epiduro™: AI-Driven Flexible Robotics Enabling Next-Generation Epidural Procedures
In recent years, we have seen many changes in the spinal implant market, including the wide range of technology now available for spine surgery. We are, of course, referring to navigation systems, robots, augmented-reality glasses, and advanced imaging technologies that undoubtedly make spine surgery both safer and more efficient. One example is Epiduro™, a new robotic … [Read more...] about Epiduro™: AI-Driven Flexible Robotics Enabling Next-Generation Epidural Procedures
Scientists Develop AxioMed Spinal Disc Replacement That Truly Mimics Healthy Human Disc — A Game-Changer in Spine Surgery
Landmark biomechanical study shows AxioMed's viscoelastic lumbar disc is the first implant to replicate the natural mechanical behaviors of a human disc, unlocking a new era of motion-preserving spine care. FORT LAUDERDALE, Fla., Nov. 18, 2025 /PRNewswire/ -- A groundbreaking scientific milestone has been achieved in spine surgery with the publication of a new peer-reviewed … [Read more...] about Scientists Develop AxioMed Spinal Disc Replacement That Truly Mimics Healthy Human Disc — A Game-Changer in Spine Surgery
The End of an Era: The Former LDR Medical Site in Troyes Faces Permanent Closure
As recently reported by Le Parisien, the historic LDR Medical production site in Troyes, France, will permanently shut down following a decision made by its new U.S. owner, Highridge Medical. The announcement, delivered to employees on September 22, came as a major shock to the workforce, which had not anticipated such a drastic shift in the company’s direction. A Success … [Read more...] about The End of an Era: The Former LDR Medical Site in Troyes Faces Permanent Closure
Medtronic reports strong second quarter fiscal 2026 financial results, enterprise growth drivers accelerate momentum
Cardiac Ablation Solutions growth of 71% on strength of pulsed field ablation (PFA) portfolio; Raising FY26 revenue and EPS guidance GALWAY, Ireland, Nov. 18, 2025 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for its second quarter (Q2) of fiscal year 2026 (FY26), which ended October 24, 2025. Q2 … [Read more...] about Medtronic reports strong second quarter fiscal 2026 financial results, enterprise growth drivers accelerate momentum
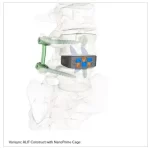
Zavation Medical Products Announces First Implantations of Varisync® ALIF NanoPrime™
FLOWOOD, Miss., Nov. 15, 2025 /PRNewswire/ -- Zavation Medical Products, LLC ("Zavation"), a leading innovator in spinal device technology, announces the first successful implantations of the Varisync® ALIF NanoPrime™ System, a next-generation stand-alone ALIF system featuring Zavation's proprietary titanium ion bond … [Read more...] about Zavation Medical Products Announces First Implantations of Varisync® ALIF NanoPrime™
Zavation Medical Products Announces NanoPrime™: A Breakthrough in PEEK and Titanium Hybrid Interbodies
FLOWOOD, Miss., Nov. 14, 2025 /PRNewswire/ -- Zavation Medical Products, LLC ("Zavation"), a leading innovator in spinal device technology, proudly announces the launch of NanoPrime™, a next-generation implant material that combines the mechanical advantages of PEEK with the biological benefits of titanium through advanced titanium ion bond … [Read more...] about Zavation Medical Products Announces NanoPrime™: A Breakthrough in PEEK and Titanium Hybrid Interbodies
Curiteva Proudly Announces the Publication of a Peer-Reviewed Study in the Journal of Biomaterials (JBI)
HUNTSVILLE, Ala., November 13, 2025 (Newswire.com) - Curiteva, Inc. a privately held manufacturing and technology company, proudly announces the publication of a peer-reviewed study, "Novel PEEK fabrication using fused strand deposition reduces inflammation and enhances MSC differentiation promoting bone growth and implant osseointegration," in the Journal of … [Read more...] about Curiteva Proudly Announces the Publication of a Peer-Reviewed Study in the Journal of Biomaterials (JBI)
OnPoint Surgical, Inc. Appoints Jeremy Laynor as Chief Commercial Officer to Accelerate Global Growth
BOSTON, Nov. 12, 2025 /PRNewswire/ -- OnPoint Surgical, Inc., a leader in Augmented Reality (AR) and Artificial Intelligence (AI) guidance for spinal interventions, today announced the appointment of Jeremy Laynor as Chief Commercial Officer (CCO). Mr. Laynor brings over two decades of executive leadership experience in the medical device, software, and … [Read more...] about OnPoint Surgical, Inc. Appoints Jeremy Laynor as Chief Commercial Officer to Accelerate Global Growth
HAPPE Spine Reaches 1,000 Patients with the INTEGRATE®-C Interbody Fusion System
GRAND RAPIDS, Mich., Nov. 13, 2025 /PRNewswire/ -- HAPPE Spine, an early stage company advancing clinical outcomes with a proprietary biomaterial and manufacturing platform for creating bone-integrating implants, announces that the INTEGRATE®-C Interbody Fusion System has been implanted in more than 1,000 patients, demonstrating clinical adoption of the … [Read more...] about HAPPE Spine Reaches 1,000 Patients with the INTEGRATE®-C Interbody Fusion System
icotec Receives FDA Clearance for the CMORE® CT System, Expanding its BlackArmor® Engineered Carbon/PEEK Technology to the Posterior Cervicothoracic Spine
ALTSTÄTTEN, Switzerland and BOSTON, Nov. 13, 2025 /PRNewswire/ -- icotec, the pioneer of implantable devices made from BlackArmor® Engineered Carbon/PEEK, today announced that the U.S. Food and Drug Administration (FDA) has cleared the CMORE® CT System for use in the cervicothoracic spine. The CMORE® (CT) System is an enhanced set of … [Read more...] about icotec Receives FDA Clearance for the CMORE® CT System, Expanding its BlackArmor® Engineered Carbon/PEEK Technology to the Posterior Cervicothoracic Spine
Centinel Spine® Surpasses 300,000 prodisc® Implantations, Reinforcing Its Leadership in Total Disc Replacement
WEST CHESTER, Pa., Nov. 13, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced that more than 300,000 … [Read more...] about Centinel Spine® Surpasses 300,000 prodisc® Implantations, Reinforcing Its Leadership in Total Disc Replacement
Spine Wave Announces Initial Surgical Procedures with the Testa™ TP Pivoting Spacer System featuring TiCell® Nano Advanced Surface Technology
SHELTON, Conn., Nov. 13, 2025 (GLOBE NEWSWIRE) -- Spine Wave is pleased to announce the initial surgical procedures benefiting from Spine Wave’s Testa™ TP Pivoting Spacer System featuring TiCell® Nano Advanced Surface Technology. These initial procedures were performed by Alexander R. Vaccaro, M.D., Ph.D., MBA¹ of Rothman Orthopaedics at Jefferson Health, Philadelphia, PA., and … [Read more...] about Spine Wave Announces Initial Surgical Procedures with the Testa™ TP Pivoting Spacer System featuring TiCell® Nano Advanced Surface Technology