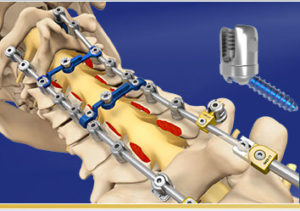
CARLSBAD, Calif., Dec. 30, 2019 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of the Mariner® Midline Posterior Fixation System. Mariner Midline is a comprehensive, less invasive posterior fixation system built upon the Mariner […]
LAUNCHES
Globus Medical launch the AERIAL™ Interspinous Fixation System
Globus Medical is excited to announce the launch of the AERIAL™ Interspinous Fixation System. AERIAL™’s easy insertion and expansion provides a simple, minimally invasive solution for interspinous fixation. AERIAL™ Interspinous Fixation is a minimally invasive spinous process fixation system. With its expandable core and independent locking plates, AERIAL™ offers a customized patient fit and allows for indirect […]
Atlas Spine Inc. completes alpha phase and announces the full launch of its first-to-market HiJak™ AC Expandable Cervical IBFD
JUPITER, FL, Feb. 27, 2019— Atlas Spine Inc., a spinal implant company based in Jupiter Florida, announced today the successful completion of its 50th surgical procedure and over 100 devices implanted with its new HiJak AC Expandable Cervical Inter-body Fusion Device (IBFD). The company has now moved to its full product launch. The HiJak AC […]
Alphatec Announces FDA Clearance of its Automated SafeOp Neuromonitoring System to Address Significant Unmet Needs in Spine Surgery
CARLSBAD, Calif., Feb. 25, 2019 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC) announced today that it has received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for its automated SafeOp neuromonitoring system for use in real-time intraoperative nerve location and health assessment. “I could not be more excited […]
ulrich medical USA® Announces Market Entry and First Global Implantation of New Generation Expandable Implant Technology
ST. LOUIS, Jan. 11, 2019 /PRNewswire/ — ulrich medical USA, Inc., a medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, announced today the market entry of a highly-anticipated, new generation vertebral body replacement device which is the company’s flagship technology in the U.S. spine implant market. The Solidity […]
Globus Medical Launches Cement Augmented Pedicle Screw System
AUDUBON, Pa., June 13, 2018 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, today announced the launch of the CREO® Fenestrated Screw System used in the treatment of patients with advanced stage tumors of the thoracolumbar spine. CREO® fenestrated screws, when augmented with the company’s FORTRESS™ radiopaque bone cement, are designed […]
NuVasive Launches AttraX Scaffold Biologic And Reports First Clinical Use
SAN DIEGO, June 12, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced the U.S. launch of AttraX® Scaffold, an absorbent ceramic-collagen bone graft with an optimized surface that has been validated in preclinical testing to drive increased bone formation and faster fusion […]
RTI Surgical® Announces Commercial Launch of Fortilink®-TS and -L IBF Systems with TETRAfuse® 3D Technology
ALACHUA, Fla.–RTI Surgical, Inc. (Nasdaq: RTIX), a global surgical implant company, announced the full commercial launch of its Fortilink®-TS and -L IBF Systems, adding to a growing series of interbody fusion devices featuring proprietary TETRAfuse® 3D Technology. The Fortilink-TS and -L Systems are intended for use in lumbar interbody fusion procedures at one or two […]
Astura Medical Announces Initial Cases and Full Commercial Release For BRIDALVEIL OCT Stabilization System
CARLSBAD, CA – February 21, 2018 – Astura Medical, a high growth, innovative spine technology company, announced today the completion of the initial surgeries and full commercial release for its BRIDALVEIL Occipital-Cervico-Thoracic (OCT) System. The first cases were successfully completed at multiple hospitals across the country, including at the University of Colorado Hospital by Dr. […]
Spine Wave Launches Proficient® Posterior Cervical Spine System Implants for Complex Posterior Cervical-Thoracic Procedures
SHELTON, Conn., Feb. 21, 2018 (GLOBE NEWSWIRE) — Spine Wave is increasing the Proficient® Posterior Cervical Spine System’s product offering to further serve the posterior cervical spine market. The system is gaining rapid adoption by spine surgeons, primarily due to its patented tri-lobe polyaxial screw design which offers a best in class 120 degrees of […]
SeaSpine Expands Ventura™ NanoMetalene® Implant Offering to Accommodate Larger Range of Posterior Procedures
CARLSBAD, Calif., Jan. 31, 2018 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ:SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the portfolio expansion of the Ventura™ NanoMetalene® posterior interbody device. Ventura NanoMetalene, which has been commercially available since 2015, has expanded its size offerings to accommodate […]
4WEB Medical Announces Launch of Next Generation Anterior Spine Truss System
DALLAS, Oct. 4, 2017 /PRNewswire/ — 4WEB Medical, the industry leader in 3D printed implant technology, has announced the launch of its next generation interbody fusion product line for anterior lumbar spine procedures. The new release of the Anterior Spine Truss System recently received FDA clearance for several impactful line extensions along with some key new indications for […]
Zimmer Biomet Announces U.S. Launch of Avenue® T TLIF Cage with Integrated VerteBRIDGE® Plating
WARSAW, Ind., Oct. 2, 2017 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced it is officially launching in the United States the Avenue® T TLIF Cage. Avenue T advances posterior lumbar cage technology by incorporating VerteBRIDGE® plating, which facilitates simplified cage insertion and zero-profile, intradiscal fixation through a direct, minimally invasive surgical […]
Medtronic Launches Orthopedic Solutions Business to Help Providers Deliver Outcome-Focused Care
DUBLIN and DALLAS – Nov. 10, 2016 – Medtronic plc (NYSE: MDT) today announced the launch of Medtronic Orthopedic Solutions, a comprehensive offering for total joint replacement episodes of care designed to drive clinical and economic outcomes. This offering is in response to the high costs associated with hip and knee replacements, along with the […]