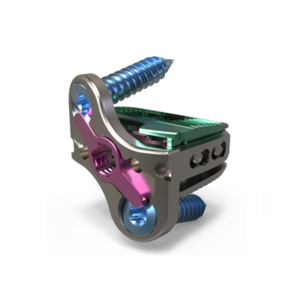
CARLSBAD, Calif., Dec. 02, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch and subsequent first case of its Admiral ACP System. Admiral ACP represents the next generation of anterior cervical plating designed to strike […]
LAUNCHES
Aurora Spine Announces Commercial Launch of The SOLO™ System
CARLSBAD, Calif., Nov. 02, 2020 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has commercially launched its proprietary SOLOTM ALIF Stand-Alone Cage system to its innovative suite of MIS products used in spinal surgery. As […]
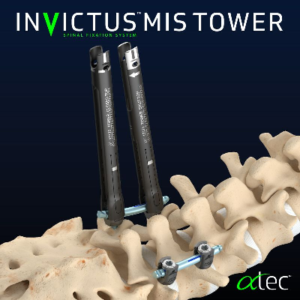
ATEC Extends InVictus MIS Platform With Commercial Release of InVictus MIS Tower
CARLSBAD, Calif., Oct. 19, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today the commercial release of the InVictus™ MIS Tower, an elegant and intuitive system designed for minimally invasive percutaneous fixation. InVictus MIS Tower is the next evolution of InVictus™, […]
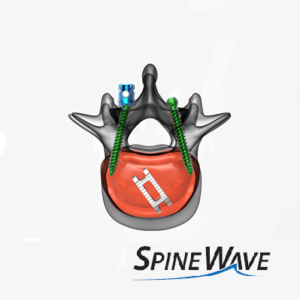
Spine Wave Announces the Commercial Launch of the Salvo® 4.75 mm Spine System
SHELTON, Conn., Oct. 15, 2020 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the successful completion of its limited market release and the launch of the Salvo® 4.75 mm Spine System, and with it, the company’s expansion into the market for thoracolumbar spinal fixation systems featuring modular screw design. The Salvo® 4.75 mm Spine System will […]
Stryker announced the full launch of the Niagara Lateral Access System
Stryker unveils their latest addition to its comprehensive minimally invasive portfolio for lateral spine surgery. Today, Stryker announced the full launch of the Niagara Lateral Access System following the completion of 600 surgeries.The company will showcase its lateral portfolio during the virtual North American Spine Society (NASS) Annual Meeting, Oct. 6-9, 2020. Niagara Lateral Access […]
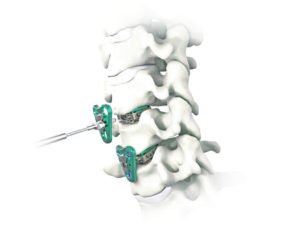
ATEC Announces Commercial Release of Distinct, Comprehensive TLIF Approach
CARLSBAD, Calif., Oct. 06, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today the commercial releases of two new solutions designed to elevate the ATEC transforaminal lumbar interbody fusion (TLIF) clinical experience. The Sigma Access System and the […]
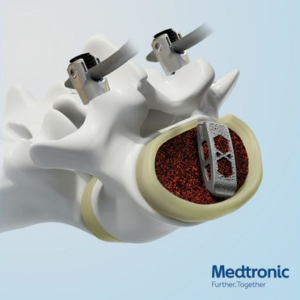
Medtronic Announces Adaptix™ Interbody System, the First Navigated Titanium Cage with Titan nanoLOCK™ Surface Technology
DUBLIN, Oct. 7, 2020 /PRNewswire/ — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. launch of Adaptix™ Interbody System, the first navigated titanium implant with Titan nanoLOCK™ Surface Technology, a proprietary blend of surface textures on the macro, micro, and nano levels. The Adaptix Interbody System, mirrored after the veteran Capstone™ Spinal […]
4WEB Medical Announces Launch of Stand-Alone Anterior Lumbar Spine Truss System
DALLAS, Oct. 6, 2020 /PRNewswire/ — 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced the commercial launch of the newest addition to the company’s spine implant portfolio, the Stand-Alone Anterior Spine Truss System™ (ASTS-SA). The first procedure was performed by James Lynch, MD, Neurosurgeon and CEO of Spine Nevada, […]
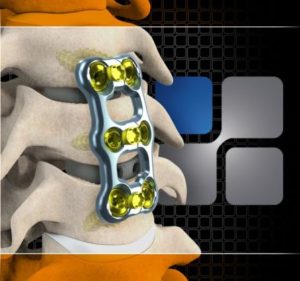
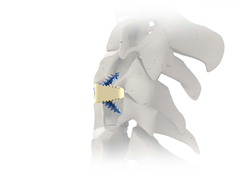
NeuroStructures Launches Transept™ Cervical Plating System
Transept™ Cervical Plating System was designed based upon an emerging trend in the peer reviewed literature identifying a significant decrease in moderate to severe Adjacent Level Ossification when the plate to disc distance is greater than 5mm from the adjacent level. The plate has an ultra-thin profile and comes with a wide spectrum of fixed, […]
Spine Wave Announces the Commercial Launch of Tornado® Bioactive Bone Graft Matrix
SHELTON, Conn., Sept. 24, 2020 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the commercial launch of Tornado® Bioactive Bone Graft Matrix, effective immediately. Tornado® Bioactive Bone Graft Matrix strengthens the company’s growing spinal biologics portfolio and marks Spine Wave’s entry into the market for synthetic bioactive bone graft matrix implants. Tornado® Bioactive Bone Graft Matrix complements […]
Medtronic Launches ADAPTIX Interbody Cage in Europe
Medtronic launches in Europe the ADAPTIX Interbody system. It is the first fully navigated TLIF device to feature the Titan NanoLOCK Surface Technology. The Adaptix™ Interbody System with Titan nanoLOCK™ Surface Technology consists of additive manufactured titanium cages of various lengths and heights to accommodate patient anatomy. The interbody device has been treated with Titan […]
Zavation Medical Products, LLC., Launches Titanium/PEEK Posterior LEIF Cage
JACKSON, Miss., Aug. 25, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of the Titanium/PEEK Posterior LEIF (Lateral Expandable Interbody Fusion) cage, a cage designed for use in the lumbar spine as a spinal fusion […]
Atlas Spine Announces Launch of its HiJAK SA Expandable Cervical Stand-Alone Interbody System
JUPITER, FL, August 19, 2020 — Atlas Spine, Inc., a spinal implant company based in Jupiter Florida, announced today the launchof its HiJAK SA Expandable Cervical Stand-Alone Interbody system, the company’s second first-to-market expandable system in just 18 months. The first implantation of the HiJAK SA Expandable Stand–Alone was performed by board certified neurosurgeon, Grant […]
SeaSpine® Announces Limited Commercial Launch of Explorer™ TO Expandable Interbody Device
CARLSBAD, Calif., Aug. 11, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch of the Explorer™ TO System. Explorer TO represents SeaSpine’s first new product offering since 2017 in the estimated $400 million expandable interbody […]
LES Spine Innovations announces launch of the new A-CIFT SoloFuse HYPER-LORDOTIC Implant offerings
BOSTON (PRWEB) AUGUST 08, 2020 LES Spine Innovations and Sagitechnology are proud to announce the launch of the new A-CIFT SoloFuse Hyperlordotic Cervical Standalone Interbody System, the first ever HA impregnated standalone cervical cage for greater sagittal correction. The A-CIFT SoloFuse Hyperlordotic Interbody is a Less Exposure Surgery (LES®) technology. LES technologies are designed with outpatient surgery in […]
ulrich medical USA® Brings Strong Momentum® to U.S. Product Launch
ST. LOUIS, July 13, 2020 /PRNewswire/ — ulrich medical USA, Inc., a medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States announced the nationwide commercial release of the Momentum Posterior Spinal Fixation System. “After completing hundreds of procedures during the alpha launch, Momentum has already made its mark with an enthusiastic reception and positive reviews from our alpha surgeon […]
SeaSpine®Announces Full Commercial Launch of Mariner® MIS and Mariner Outrigger™ Spinal Fixation Systems
CARLSBAD, CA (June 30, 2020) – SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launches of Mariner MIS and Mariner Outrigger. “The commercial launches of Mariner® MIS and Mariner Outrigger™ result from meticulous product refinement and creative application of differentiated […]
SeaSpine® Announces Limited Commercial Launches of NorthStar™ OCT and Cervical Facet Fusion™ Systems
CARLSBAD, CA (June 22, 2020) – SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launches and completion of initial surgeries of both its NorthStar OCT and Cervical Facet Fusion systems, which significantly expand its procedural offerings for posterior […]
NuVasive Expands Complex Spine Portfolio with Global Launch of Reline 3D for Pediatric Deformity
SAN DIEGO, June 18, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the expansion of its complex spine portfolio with the global commercial availability of Reline 3D™, a posterior fixation system for patients suffering from pediatric spinal deformities. The Reline […]
Spinal Elements® Introduces its MIS Ultra™ Platform of Products and Procedures Designed to Minimize the Unintended Consequences of Spine Surgery
Carlsbad, CA – June 17, 2020 – Spinal Elements, a Carlsbad, CA-based medical device company focused on spine surgery procedures, today introduced its MIS Ultra™ platform of products and procedures. MIS Ultra is a suite of minimally invasive integrated instrument and implantable device solutions designed to optimize spinal fixation and restoration while minimizing the unintended […]
Zavation Medical Products, LLC, Launches Cortical Screw
JACKSON, Miss., June 16, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of the Cortical Screw, a screw designed for use in the cortical bone as an extension of the Zavation Spinal System. The Cortical […]
OrthoPediatrics Corp. Commences Initial U.S. Launch of ApiFix’s FDA-Approved Spinal Deformity Correction System
WARSAW, Ind., June 10, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq:KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, is pleased to announce the initial U.S. launch of the ApiFix Minimally Invasive Deformity Correction (“ApiFix”) system. Additionally, the Company anticipates approximately 20 leading clinical centers in the United States to […]
Zavation Medical Products, LLC. Launches eZspand™ Expandable Cage
JACKSON, Miss., May 26, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of eZspand™, an expandable lumbar interbody fusion device. eZspand™, the latest addition to Zavation’s portfolio, features unmatched expandable precision paired with continual expansion […]
Camber Spine Technologies Announces Nationwide Launch of SPIRA®-C Integrated and FORTICO™ Anterior Cervical Plate
KING OF PRUSSIA, Pa., May 7, 2020 /PRNewswire/ — Camber Spine, a leading innovator in spine and medical technologies, today announced the FDA clearance and nationwide launch for two novel anterior cervical products: The SPIRA®– C Integrated Interbody system, a stand-alone integrated fixation system, and the FORTICO™ Anterior Cervical Plating System, a two screw plating system intended for […]
Genesys Spine is pleased to announce the launch of a Sacroiliac Joint Fusion System.
Thursday, February 20, 2020–Austin, TX: The Genesys Spine Sacroiliac Joint Fusion System consists of partially threaded and fully threaded implants designed to secure the sacroiliac joint and minimize micro-motion enabling bony fusion. Advantages of the Genesys Spine Sacroiliac Joint Fusion System include: Dual thread designs incorporate a differential pitch for controlled compression across the joint. […]
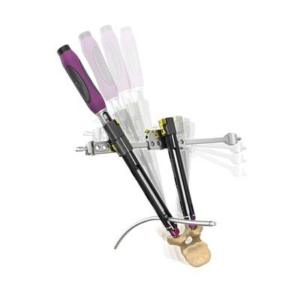
Atlas Spine Launches its V3 Guided Segmental Cervical Plating System, Expands Solutions for Cervical Deformity
Palm Beach County, FL, January 28, 2020 — Atlas Spine Inc., a spinal implant company based in Jupiter, Florida, announced today the launch of its V3 Guided Segmental Plating System, expanding the company’s disruptive technology solutions for treating complex deformity and degenerative conditions of the cervical spine. Cervical spine pathologies afflict hundreds of thousands of people each year. The general growth of an aging population […]
SpineCraft announces the launch of the ASTRA Occipito-Cervico-Thoracic (OCT) Spine System
January 24, 2020 –The ASTRA-OCT System is a comprehensive set of implants and instruments to stabilize the spine in patients undergoing posterior cervical fusion. The system was developed to address unmet needs in complex posterior spine fixation procedures, revisions and extensions. ASTRA-OCT includes a wide range of screw, connector and rod options for both the […]