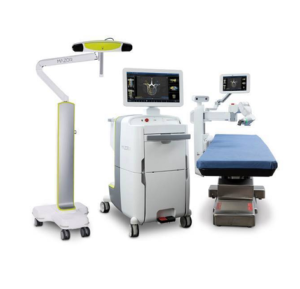
LAS VEGAS–(BUSINESS WIRE)–Celebrating its 20th anniversary in 2019, Medacta International is showcasing its robust personalized medicine portfolio this week at the American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting. The recently unveiled Medacta Individualized Kinematic Alignment (MIKA) instrumentation and associated surgical technique, MectaLIF® Anterior Interbody Fusion Device, and MySpine® MC Surgical Guides, among other key […]
NEWS
BIOMECH Paonan to Showcase NEST Interbody System at AAOS 2019
BIOMECH Paonan will feature their new NEST Interbody System and highlight its Paonan’s 3D printing Titanium technology at the American Academy of Orthopaedic Surgeons Annual Meeting March. 13–15, 2019, in Las Vegas (booth No. 3755). During the AAOS, BIOMECH Paonan will also exhibit all their spinal products as well as other product categories including their […]
Smith & Nephew Acquires Brainlab Orthopaedic Joint Reconstruction Business
LONDON, March 12, 2019 /PRNewswire/ –Brainlab, the global pioneer of software-driven medical technology, and Smith & Nephew (LSE: SN,NYSE: SNN), the global medical technology business, announce that Smith & Nephew has agreed to acquire the Brainlab orthopaedic joint reconstruction business. The acquisition supports Smith & Nephew’s strategy to invest in best in class technologies that further its multi-asset digital […]
Kuros Biosciences Receives US Marketing Clearance for Intervertebral Body Fusion Device
March 13, 2019 02:00 ET | Source: Kuros Biosciences SCHLIEREN (ZURICH), Switzerland, March 13, 2019 (GLOBE NEWSWIRE) — Kuros Biosciences (SIX: KURN) today announced that its Dutch subsidiary, Kuros Biosciences BV, has received clearance for the Kuros TLIF cage from the U.S. Food and Drug Administration (FDA). The TLIF cage has been developed for the use with KUR-113, […]
Tyber Medical, LLC Awarded Competitive Grant from the National Science Foundation
BETHLEHEM, Pa., March 12, 2019 /PRNewswire/ — Tyber Medical, LLC has been awarded a National Science Foundation (NSF) Small Business Innovation Research (SBIR) grant for $225,000 to conduct research and development (R&D) work aimed at reducing the incidence of orthopedic surgical site infections (SSI). To address the severe clinical and economical effects of orthopedic device-related infections, Tyber Medical is aggressively exploring the antibacterial […]
joimax® Hosts International Training Summit to Showcase Innovative Technologies
KARLSRUHE, Germany–(BUSINESS WIRE)–Earlier this month, at the international training summit dubbed Faculty and User Meeting, joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, presented its newest products and technologies. During the two-day event, joimax® also honored four surgeons with the Kambin Award and introduced the Endoscopic Spine Academy, […]
Kyocera to Showcase New Products at AAOS 2019, Including Assets from Renovis Surgical
AUSTIN, Texas–(BUSINESS WIRE)–Kyocera Medical Technologies, Inc. announced today that it will exhibit its latest products and technologies at the American Academy of Orthopedic Surgeons (AAOS) 2019 annual meeting, March 13-16 in Las Vegas. Kyocera’s U.S. orthopedic market presence has expanded substantially this year with its purchase of the major assets and intellectual property portfolio of […]
RTI Surgical® Announces Appointment of Jeffrey C. Lightcap to Board of Directors
DEERFIELD, Ill., March 08, 2019 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, today announced the appointment of Jeffrey C. Lightcap to its Board of Directors, effective immediately. Mr. Lightcap has significant experience in helping drive growth in medical technology companies. He is currently a Senior Managing Director at HealthCor Partners Management, L.P., a growth equity investor focused […]
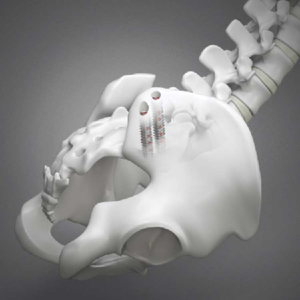
Why the MIS Sacroiliac Joint Fusion market will reach from US$ 150 million in 2019.Which are the Key players?
According to MarketWatch, over the next five years the MIS Sacroiliac Joint Fusion market will register a 15.5% CAGR in terms of revenue, the global market size will reach US$ 360 million by 2024, from US$ 150 million in 2019. According to iData Research the U.S. minimally invasive spinal implants market is expected to increase […]
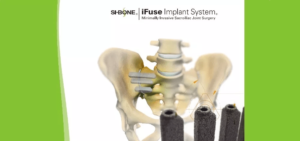
SI-BONE, Inc. Announces Journal of Bone and Joint Surgery (JBJS) Publishes 2-Year Results from iMIA, a European Multicenter Randomized Controlled Trial (RCT) of SI Joint Fusion with iFuse Implant System® vs Conservative Care
SANTA CLARA, Calif., March 06, 2019 (GLOBE NEWSWIRE) — SI-BONE, Inc. (Nasdaq: SIBN), a medical device company that pioneered minimally invasive surgery of the sacroiliac (SI) joint with the iFuse Implant System® (iFuse), announced publication of 2-year results from iMIA (iFuse Implant System Minimally Invasive Arthrodesis; ClinicalTrials.gov ID NCT01741025), a multicenter European RCT in the Journal of Bone and Joint Surgery […]
Alphatec Spine Reports Fourth Quarter and Full Year 2018 Financial Results
CARLSBAD, Calif., March 07, 2019 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), today announced financial results and operating highlights for the fourth quarter and full year ended December 31, 2018. The Company also announced that it has secured an additional $30 million financing commitment from Squadron Capital to fund continued […]
RTI Surgical® Shareholders Approve Paradigm Spine Acquisition
ALACHUA, Fla., March 07, 2019 (GLOBE NEWSWIRE) — RTI Surgical, Inc. (Nasdaq: RTIX), a global surgical implant company, today announced its shareholders have voted to approve completion of the company’s previously announced acquisition of Paradigm Spine, LLC, a leader in motion preservation and non-fusion spinal implant technology. The final voting results have been disclosed on […]
Ryan J. Dickinson Named Vice President Of Sales And New Business Development For Camber Spine
KING OF PRUSSIA, Pa., March 7, 2019 /PRNewswire/ — Camber Spine, a leading innovator in spine and medical technologies, today reported Ryan J. Dickinson named Vice President of Sales and New Business Development for Camber Spine. Camber Spine is pleased to announce the appointment of Ryan J. Dickinson to the position of Vice President of Sales and New Business Development. Ryan […]
SpineGuard Granted Patent for Ultrasound Mapping of the Spine
SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that deploys its DSG® real time digital technology for surgical guidance intended to secure and streamline skeletal implant placement, announced today the grant of a patent for the mapping of bony structures by ultrasound, in France and Japan. The patent discloses a novel manner to use ultrasound […]
Simplify Medical Announces 60th U.S. Patent for Innovative Cervical Disc Replacement Portfolio
SUNNYVALE, Calif., March 07, 2019 (GLOBE NEWSWIRE) — Simplify Medical Pty Ltd., maker of the Simplify® cervical artificial disc, today announced receipt of the company’s 60th U.S. patent. The 60th patent, U.S. Patent No. 10,219,911, is entitled “Prosthetic Disc for Intervertebral Insertion” and relates to a method for assembling a cervical disc replacement in which […]
MCRA Assists with Premarket Approval of the M6-C Artificial Cervical Disc
WASHINGTON, March 7, 2019 /PRNewswire/ — MCRA, LLC announced today its role in the successful Premarket Approval (PMA) application decision by the U.S. Food and Drug Administration (FDA) to approve the Orthofix M6-C™ artificial cervical disc for the treatment of single level cervical disc degeneration. A next-generation artificial cervical disc, the M6-C disc developed by […]
NuVasive To Feature Lateral Single-Position Surgery And Surgical Intelligence At Spine Summit 2019
SAN DIEGO, March 6, 2019 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced its participation in and sponsorship of Spine Summit 2019 – 35th Annual Meeting of the Section on Disorders of the Spine and Peripheral Nerves, held March 14–17, 2019 at the […]
Cerapedics Announces ISASS Bone Grafting Policy Statement Features i-FACTOR™ Peptide Enhanced Bone Graft
WESTMINSTER, Colo., March 6, 2019 /PRNewswire/ –Cerapedics, a privately-held orthobiologics company, today announced that the International Society for the Advancement of Spine Surgery (ISASS) issued a new bone grafting policy with recommendations on usage and payor coverage criterion that features i‑FACTOR™ Peptide Enhanced Bone Graft as one of only two drug-device combination products approved by the U.S. […]
Amplify™ Surgical, Inc. Announces First Surgical Cases with dualX™ Dual Expanding Interbody Fusion System
MISSION VIEJO, CALIF. (PRWEB) MARCH 06, 2019–Amplify Surgical, Inc., a privately-held spinal device company, focusing on the development and commercialization of minimally-invasive, innovative spinal technologies, today announced the completion of several surgical cases with the dualX Dual Expanding Interbody Fusion System. The first two lateral lumbar interbody fusion (LLIF) procedures implanting dualX were performed by two […]
Intralink-Spine, Inc. Goes Down Under To Extend Clinical Studies
LEXINGTON, Ky.–(BUSINESS WIRE)–Today, Intralink-Spine, Inc. announces the initiation of clinical trials in Australia for their patented medical device for the treatment of low back pain, called Réjuve™. “We’re excited that patient enrollment has begun in Australia. The physicians and staffs involved in the Réjuve clinical safety study (GEM-SE) are top-notch,” says Lyle Hawkins, CEO, of Intralink-Spine, Inc. “There […]
Are Interspinous Fusion Devices a real alternative to Pedicle Screw Fixation?Which Are the Competitors?
Interspinous fusion devices were developed to aid in the stabilization of the spine as alternatives to pedicle screw fixation in combination with interbody fusion.But are the interspinous Fusion devices a real alternative to pedicle screw fixation? There are two main concerns: Primary fixation*: Since these devices attach to the spinous processes there is the risk […]
Globus Medical launch the AERIAL™ Interspinous Fixation System
Globus Medical is excited to announce the launch of the AERIAL™ Interspinous Fixation System. AERIAL™’s easy insertion and expansion provides a simple, minimally invasive solution for interspinous fixation. AERIAL™ Interspinous Fixation is a minimally invasive spinous process fixation system. With its expandable core and independent locking plates, AERIAL™ offers a customized patient fit and allows for indirect […]
Nexxt Spine to use MTS material test systems to develop 3D-printed bone healing spinal implants
Spine, an Indiana-based spinal implants manufacturer, has announced that it will develop 3D printed bone healing spinal implants with the help of MTS Systems Corporation, a global manufacturer and supplier of simulations and testing systems. Alaedeen Abu-Mulaweh, Nexxt Spine Director of Engineering, said, “Given the highly nuanced nature of these intricate micro geometries, the MTS testing […]
Medtronic Mazor X robotic surgery system enters medical mainstream
(Star Tribune)– Dr. Eiman Shafa of the Twin Cities Spine Center, left, demonstrated the use of Medtronic’s Mazor X robotically navigated spine-surgery system Tuesday at Abbott Northwestern Hospital. Following years of work, Medtronic is launching the newest Mazor X spinal surgery system with robotic navigation in the United States, with two systems already sold in […]
Medtronic Announces Approval and Launch of Japan’s First DBM Bone Grafting Product for Spine and Orthopedic Procedures
DUBLIN and TOKYO – February 28, 2019 – Medtronic plc (NYSE:MDT) announced today the Japanese launch of the Grafton(TM) Demineralized Bone Matrix (DBM) bone grafting product for spine and orthopedic procedures. Grafton DBM is the first and only demineralized bone matrix product available in Japan, the world’s second largest market for spinal medical devices. Due to […]
RTI Surgical® Announces Fourth Quarter and Full Year 2018 Results
ALACHUA, Fla., Feb. 28, 2019 (GLOBE NEWSWIRE) — RTI Surgical, Inc. (Nasdaq: RTIX), a global surgical implant company, reported operating results for the fourth quarter and full year of 2018. Fourth Quarter 2018 Highlights: Revenue increased to $71.2 million Net income of $2.1 million, inclusive of $1.0 million of net non-recurring expenses Adjusted EBITDA of […]
Atlas Spine Inc. completes alpha phase and announces the full launch of its first-to-market HiJak™ AC Expandable Cervical IBFD
JUPITER, FL, Feb. 27, 2019— Atlas Spine Inc., a spinal implant company based in Jupiter Florida, announced today the successful completion of its 50th surgical procedure and over 100 devices implanted with its new HiJak AC Expandable Cervical Inter-body Fusion Device (IBFD). The company has now moved to its full product launch. The HiJak AC […]