The coflex Interlaminar Technology is an Interlaminar Stabilization™ device indicated for use in one or two level lumbar stenosis from L1-L5 in skeletally mature patients with at least moderate impairment in function, who experience relief in flexion from their symptoms of leg/buttocks/groin pain, with or without back pain, and who have undergone at least 6 months of … [Read more...] about Coflex Interlaminar
Interspinous
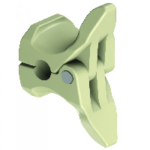
Giglio Interspinous Fusion System
By using Tsunami Medical’s cutting-edge titanium net structure in combination with the geometry of the implant, Giglio differentiates from the alternatives by its accelerated osteogenesis characteristics. Primary fixation is assured by accurate sizing in the interspinous space in combination with expandable wings. After implantation and correct positioning of the implant, the … [Read more...] about Giglio Interspinous Fusion System
InsEx
LfC is one of few spinal implant developers worldwide, who can be proud of launching on the market „next generation” of interspinous implants. Long-term international experience with previous solutions (InterS/inSWing) and many consultations with surgeons resulted in development of the breakthrough solution, but most of all, one that fullfills surgeons … [Read more...] about InsEx
AERIAL™ Interspinous Fixation
AERIAL™ Interspinous Fixation is a minimally invasive spinous process fixation system. With its expandable core and independent locking plates, AERIAL™ offers a customized patient fit and allows for indirect decompression. AERIAL™’s easy insertion and expansion provides a simple MIS solution for interspinous fixation. AERIAL VIDEO … [Read more...] about AERIAL™ Interspinous Fixation
Alpine XC Adjustable MIS Fusion System
The Alpine XC Adjustable MIS Fusion System is a posterior attachment spinal fixation system compos/ed of spinous process plates, dedicated surgical instruments, and sterilization cases. The components are used to build a construct to provide stabilization of spinal segments in the thoracic, lumbar and sacral spine to support fusion. The Alpine XC Adjustable MIS Fusion System … [Read more...] about Alpine XC Adjustable MIS Fusion System
AILERON-TRX
The AILERON-TRX Expandable Posterior Fusion System responds to the patient’s and surgeon’s need for a simple, reliable, and less invasive system with minimal exposure and dissection. AILERON-TRX VIDEO ANIMATION The in-situ expandable core offers patient matched expansion ensuring an ideal fit for every patient anatomy and pathology. Top Rail design increases ease … [Read more...] about AILERON-TRX
Aspen® MIS Fusion System
The Aspen MIS Fusion System consists of a family of spinous process fixation devices designed for rigid, posterior fixation to promote fusion from T1 to S1. Aspen VIDEO ANIMATION (Lanx version)Aspen-Brochure.Zimmer Biomet.pdfAspen-Surgical-Technique.Zimmer Biomet.pdf Less invasive alternative to traditional pedicle screws when used as an adjunct to interbody fusionSimple … [Read more...] about Aspen® MIS Fusion System
Axle
The Axle Interspinous Fusion System is an internal fixation device for posterior spinal surgery in the non-cervical spine (T1-S1 inclusive). It is a minimally invasive, modular Interspinous fusion system with straightforward instrumentation. The Axle Interspinous Fusion System is designed to provide spinal stability for lumbar fusion procedures, including the treatment of … [Read more...] about Axle
Affix II Mini
NuVasive® Spinous Process Plate System is a posterior, non-pedicle supplemental fixation device, intended for use in the non-cervical spine (T1 – S1).It is intended for plate fixation/attachment to spinous process for the purpose of achieving supplemental fusion in the following conditions: degenerative disc disease – defined as back pain of discogenic origin with degeneration … [Read more...] about Affix II Mini
AILERON
AILERON Posterior Fusion System attaches to the spinous processes for the purpose of achieving fusion in conjunction with bone graft. With minimal exposure and dissection, AILERON responds to the patient’s and surgeon’s need for a simple, reliable, and less invasive system. Benefits: - Less invasive approach results in minimal tissue disruption and trauma - Bullet tip … [Read more...] about AILERON
Aperius
Aperius PercLID System was the first Percutaneous stand-alone Lumbar Interspinous Decompression System. It is indicated for patients suffering from degenerative lumbar spinal stenosis with neurogenic intermittent claudication between the interspinous process spaces of L1 to L5. Aperius - Patient brochure.Medtronic.pdf Classification of Interspinous Devices :The … [Read more...] about Aperius
Benefix™ Interspinous Fixation System
Benefix™ Interspinous Fixation System is intended to provide immobilization and stabilization of the spinous processes to support fusion. The components can be assembled in a various configurations so that adaptations can be made to take into pathology and individual patient anatomy. Benefix VIDEO ANIMATION Product Feature Aggressive fixation spikes to … [Read more...] about Benefix™ Interspinous Fixation System
BridgePoint
BridgePoint is an MIS system that can replace pedicle screws for certain indications and patient types. The implant has unique telescoping plates that allow surgeons to fixate and compress spinous processes to restore sagittal alignment and facilitate a reliable interbody fusion. BridgePoint VIDEO ANIMATION The device’s large contact area provides a strong anchor point from … [Read more...] about BridgePoint
BacJac
BacJacs minimally invasive, unilateral surgical approach reduces operating room and patient recovery time, while preserving future surgical options. The BacJac is a self-deploying, non-fusion device which is tissue sparing and ligament preserving. BacJac VIDEO ANIMATION The BacJac achieves spinal decompression by limiting the symptomatic extension while maintaining … [Read more...] about BacJac
Clutch® Interspinous Process Device
The Clutch interspinous process device’s proprietary spring-activated mechanism is designed to maintain active compression against the spinous processes to resist loosening and migration. Additionally, the device’s bone opposing surfaces are enhanced with clinically proven Ti-Bond® technology, designed to provide immediate stability and long-term fixation. Clutch-Spinal … [Read more...] about Clutch® Interspinous Process Device
Coflex F
Coflex F stabilization system is a posterior, non-pedicle supplemental fixation device intended for use with an interbody cage as an adjunct to fusion at a single level in the lumbar spine (LI - SI). It is intended for attachment to the spinous processes for the purpose of achieving stabilization to promote fusion in patients with degenerative disc disease - defined as back … [Read more...] about Coflex F
DIAM™ Spinal Stabilization System
The DIAM™ implant is a dynamic spinal stabilization device that can be used to treat low back pain and radiating leg pain. It is placed in your spine, and helps share the load borne by the disc. It also opens the space between vertebrae so nerves have more of their naturally intended room. NOTE: THIS PRODUCT IS NOT CURRENTLY AVAILABLE IN THE UNITED STATES About … [Read more...] about DIAM™ Spinal Stabilization System
DynaZIP
DynaZIP is Aurora Spine's minimally invasive posterior dynamic interspinous implant for motion preservation without the need for fusion.Not yet FDA cleared nor CE Mark approved. Dynamic Stabilization:Motion preservation without the need for fusion. ZIP ONE-STEP™ locking mechanism:Secured without the use of a set screw. No torque required. Pre-packaged Sterile:Guaranteed … [Read more...] about DynaZIP
Evo
Evo is an innovative interlaminar fusion device made entirely with 3D printing technology, composed of a macrostructure in trabecular titanium that promotes rapid bone growth. Its central body in trabecular titanium – perforated both in the craniocaudal and lateral direction – allows the surgeon to insert a greater quantity of bone graft which increases the reachable area of … [Read more...] about Evo