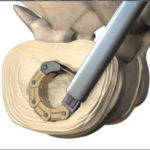
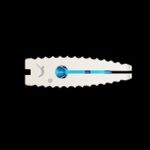
L-VARLOCK® cage is a lumbar cage made of titanium, which makes it possible to perform posterior lumbar arthrodesis (PLIF) with restoration of disc height. Benefits: About KISCO International KISCO International has developed and manufactured medical devices for the spine since 1983. Today, it is a thriving company that has become an important player in … [Read more...] about L-VARLOCK
EXPANDABLE CAGES
LorX® Expandable
LorX® Expandable PLIF Peek Cage has an unique design in various heights to restore the interbody space.Two adequate graft spaces for enhanced bone fusion.Specially designed (patent pending) expanding shaft has graft holes in the aim of filling bone graft after insertion. Benefits: Better Stability Anatomical Shape and Biocompatibility Easy … [Read more...] about LorX® Expandable
Luna 360 Interbody Fusion System
Luna XD allows an ALIF-sized, circular implant surgical delivery via a posterior, minimally-invasive access point to the intervertebral space. Once inserted, the Luna XD implant first expands horizontally to cover the disc footprint, creating a stable foundation. Then the implant expands vertically up to 16mm to restore disc height. The device includes up to 12° of lordosis to … [Read more...] about Luna 360 Interbody Fusion System
Leva® PX Interbody Device
The Leva™ Interbody Device is an expandable titanium implant that is optimized for ease of insertion and for maximum post expansion bone graft capability. The retractable bullet nose design facilitates insertion, while the instrumentation provides controlled expansion within the interbody space. Perhaps most important, following the implantation and expansion of the … [Read more...] about Leva® PX Interbody Device
LONGBOW™
The innovative LONGBOW Expandable Lateral Spacer System is the first interbody on the market that expands laterally (A/P) in-situ specifically for a direct lateral approach. The benefits of LONGBOW are transferred directly to the patient by minimizing tissue retraction and potential nerve damage associated with the lateral access approach. LONGBOW VIDEO … [Read more...] about LONGBOW™
Omega XP Expandable Lumbar Interbody Device
The Omega XP Expandable Lumbar Interbody Device is designed for use in intervertebral body spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels from L2-S1. Features: About Spinal Elements Spinal Elements, headquartered in Carlsbad, CA, is a spine technology company for spine surgeons who demand … [Read more...] about Omega XP Expandable Lumbar Interbody Device
LATIS®
LATIS® is an innovative expandable lumbar interbody fusion spacer designed to provide an ALIF footprint through a TLIF approach. Features: About Globus Medical Globus Medical, Inc. is a leading spinal implant manufacturer and is driving significant technological advancements across a complete suite of spinal products. Founded in 2003, Globus’ single-minded … [Read more...] about LATIS®
LOTUS Expandable Cage
LOTUS ™ 2-step Expandable Lumbar Interbody Fusion Cage (Expandable PLIF) with 2 steps expand feature to ease expandable pressure of endplate and prevet from loosing from endplate during surgery with 4 anti-loose tooth. About Expandable Cages Expandable interbody cages allow surgeons to go through a small corridor and change the geometry of the cage to correspond to what … [Read more...] about LOTUS Expandable Cage
MOJAVE™ PL 3D Expandable Interbody System
The MOJAVE™ PL 3D Expandable Interbody System is a fusion device designed to allow for independent control of the anterior and posterior height in the lumbar spine. Featuring infinite adjustment within the expansion range, the implant may be locked at any desired height and lordosis to aid in the restoration of sagittal balance. Designed with K2M’s Lamellar 3D … [Read more...] about MOJAVE™ PL 3D Expandable Interbody System
Opticage Expandable
The Opticage™ provides surgeons an FDA cleared and CE approved intervertebral fusion cage that can be custom fit to the patient anatomy as it is adjustable within its working range. DePuy Synthes Products, Inc., part of the Johnson & Johnson Family of Companies, announced on January 3, 2017 an asset purchase and development agreement with Interventional Spine, Inc., a … [Read more...] about Opticage Expandable
PROW FUSION™
The PROW FUSION™ for Transforaminal Lumbar Interbody Fusion (TLIF) procedures is the first clinical application of NLT SPINE’s non-linear technology platform to be introduced to the U.S. The PROW FUSION™ interbody fusion implant is composed of a segmented non-linear structure and is delivered in a straight configuration (linear) through a protective conduit that is inserted … [Read more...] about PROW FUSION™
PANGO Ⅱ Expandable Cage (TI+3D)
PANGU Ⅱ is one Lumbar Fusion Cage system, indicated for: Degenerative lumbar vertebral instability; Lumbar disc herniation or Lumbar Stenosis; Patients get post-surgical lumbar vertebral instability, while simultaneously need posterior pedicle screw fixation treatment; Patients suffer from lumbar discogenic pain and are restricted to anterior surgery; Patients … [Read more...] about PANGO Ⅱ Expandable Cage (TI+3D)
PROLIFT®
The PROLIFT Expandable Spacer System with Osseo-Loc Surface Technology, provides a Micro Invasive solution for TLIF and PLIF procedures. With up to 6mm of in situ expansion, ProLift allows for restoration of normal anatomic disc height and decompression of neural elements. Streamlined instrumentation provides the surgeon minimal tissue disruption and nerve … [Read more...] about PROLIFT®
PROLIFT MICRO Endoscopic Expandable Spacer System
ProLift Micro Expandable Spacer System with Osseo-Loc Surface Technology offers a micro invasive solution for Endoscopic Fusions. With up to 5mm of in situ expansion, ProLift allows for restoration of patient disc height and preserves anatomy. Streamlined instrumentation provides the surgeon minimal tissue disruption and nerve retraction while achieving surgical goals and … [Read more...] about PROLIFT MICRO Endoscopic Expandable Spacer System
PROCAYMAN™
PROCAYMAN is an Expandable PLIF Peek Cage. Features: About Expandable Cages Expandable interbody cages allow surgeons to go through a small corridor and change the geometry of the cage to correspond to what the patient’s spine requires. These implants may help protect a patient’s nerve roots and other structures in the area near the cage or along the corridor … [Read more...] about PROCAYMAN™
RISE®
RISE® is an all titanium expandable lumbar fusion device which minimizes insertion force, provides controlled distraction and optimizes endplate-to-endplate fit. Features: About Globus Medical Globus Medical, Inc. is a leading spinal implant manufacturer and is driving significant technological advancements across a complete suite of spinal products. … [Read more...] about RISE®
Spinal Jaxx
Spinal Jaxx is an expandable lumbar fusion device intended for use in spinal fusion surgeries and allows surgeons to customize the implant to the specific anatomy of the patient. The proprietary instrumentation also allows the surgeon to effectively fill the implant with auto graft after implantation and set the implant to the desired expansion … [Read more...] about Spinal Jaxx
StaXx® XD Expandable Device
The StaXx® XD Expandable Device is an expandable PEEK spacer that allows for controlled in situ distraction in 1mm increments. The low-profile, expandable design allows for a significantly smaller working channel compared to a standard monolithic implant, which may result in less bone removal and nerve retraction. The device is designed to be expanded in situ to allow surgeons … [Read more...] about StaXx® XD Expandable Device
StaXx IB Expandable Interbody Device
StaXx® IB is a low-profile, expandable PEEK interbody implant that allows for packing of graft material both inside and around the implant to promote fusion of the interbody space.The StaXx® IB System is an intervertebral body fusion device intended for spinal fusion procedures inside the U.S. Benefits: About Spine Wave Spine Wave was founded in February 2001 by … [Read more...] about StaXx IB Expandable Interbody Device