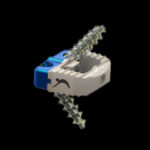
Adhesion and Visibility combine for a sophisticated solution. MATISSE™ Ti-PEEK ACIF Cage with TiCro™ technology is a versatile cervical interbody fusion device with a radiolucent PEEK cage body and adhesive Titanium End Plate Shell. Matisse-Ti-PEEK-Brochure.CTL-AMedica.pdf TiCro™ surface architecture is CTL Medical’s proprietary new approach to machined titanium … [Read more...] about MATISSE™ TI-PEEK ACIF CAGE WITH TICRO™
ACIF
Monza
Monza is an innovative cervical cage that, thanks to its trabecular titanium structure made with the most modern 3d printing techniques, guarantees immediate and safe mechanical stability and certain osseointegration to all types of implants. The cervical cage was developed to cover all possible sizes and degrees of lordosis and allows … [Read more...] about Monza
Miraclus ACC
The ACC stand alone implant System working as a Stand Alone Implant for use in cervical interbody fusion, which comprises the functionality of a cervical inter vertebral spacer and benefits of an anterior cervical plate. Features: Peek Interbody Spacer Titanium Alloy Plate Locking Screw About Miraclus At Miraclus, we innovate, manufacture and market … [Read more...] about Miraclus ACC
Modulus C
Unlike any other 3D printed titanium implant on the market, Modulus is designed through a proprietary optimization algorithm that balances strength and radiolucency. MODULUS C VIDEO ANIMATION Advanced Materials Science™Adhering to the three core principles of Advanced Materials Science—Surface, Structure, and Imaging—NuVasive has pioneered design and manufacturing methods … [Read more...] about Modulus C
Mecta-C Stand Alone Interbody Fusion
The Medacta Mecta-C Stand Alone Interbody Fusion Device offers an a MODULAR design that incorporates the benefits of an anterior plate and a radiolucent interbody spacer. In order to accommodate specific anatomical requirements and specific pathologies to treat, the surgeon has the ability to assemble any of the available plates intraoperatively giving the … [Read more...] about Mecta-C Stand Alone Interbody Fusion
Monet™ Anterior Cervical Fusion system
The Monet™ Anterior Cervical Fusion system is designed for intra-operatively flexibility. The cage component can be implanted independently or in conjunction with the Monet™ supplemental fixation plates, making it a truly comprehensive Anterior Cervical Fusion solution. Features: CTL Amedica CTL Amedica is a forward thinking medical device design, development, and … [Read more...] about Monet™ Anterior Cervical Fusion system
MONET NITRO™ anterior cervical fusion system
MONET NITRO™ anterior cervical fusion system is CTL Amedica's exclusive and enhanced addition to the NITRO™ family of interbody implants crafted entirely of the heavily researched and proven biomaterial, silicon nitride Features: CTL Amedica CTL Amedica is a forward thinking medical device design, development, and manufacturing company aiming to become the leader … [Read more...] about MONET NITRO™ anterior cervical fusion system
MINERVA
The MINERVA TM Variable Angle ACIF Cage is a stand-alone interbody fusion device with internal screw fixation and is intended to be used inanterior cervical discectomy and fusion procedures, which combines the functionality of a cervical interbody spacer and benefits of ananterior cervical plate. Minerva Cervical Cage VIDEO … [Read more...] about MINERVA
neoWave™ C
neoWave™C is a 3D-printed interbody fusion device featuring its repeating waveform matrix technology that is designed to support postoperative osseointegration while uniquely diffusing compressive loads to reduce device stiffness and, therefore, the risk of subsidence. The proprietary waveform technology also functions to disperse loads during device malleting to meet the … [Read more...] about neoWave™ C
NEXXT MATRIXX® Stand Alone Cervical System
Designed to be used as an adjunct to fusion at one or two contiguous levels (C2-T1) in skeletally mature patients, the Stand-Alone Cervical System will help patients that suffer from the treatment of degenerative disc disease. It should be used with the bone screw fixation provided and requires no additional fixation. The increasing adoption of additive manufacturing into … [Read more...] about NEXXT MATRIXX® Stand Alone Cervical System
OYSTER ACIF
State-of-the-art production technology ,3D titanium printing enables an open-pore cage design with a mesh macrostructure composed of approximatively 73% air and 27% titanium. Good primary stability thanks to teeth and spikesAvailable in anatomical as well as wedge-shaped designEasy and intuitive instrumentation and disassembly of instruments Benefits: Implant Porosity … [Read more...] about OYSTER ACIF
Optio-C® Anterior Cervical System
The Optio-C System is the industry's first no profile, modular stand-alone cervical device that offers allograft* and PEEK options and delivers the strength, stabilityand fusion potential of a traditional ACDF. Optio-C-Brochure-Zimmer Biomet.pdf Optio-C-PEEK.SGT-Zimmer Biomet.pdf Optio-C-Allograft.SGT-Zimmer-Biomet.pdf Benefits: No Profile REDUCES soft tissue … [Read more...] about Optio-C® Anterior Cervical System
OVERFIX Cervical Cage
Intersomatic cages are very useful implants among the various cervical surgical options due to their good medical results, because they eliminate the need for autografts and because of their excellent compatibility with Nuclear Magnetic Resonance. OVERFIX-SGT.Bioadvance.pdf Features: Autostatic teeth:More stability and resistance to displacement. Axial window:Important … [Read more...] about OVERFIX Cervical Cage
ONIX
The ONIX® cage system self-sustaining cervical PEEK (biocompatible polymer), comes in various heights, with an angle of 6º, and includes 2 titanium markers at the front and back end. Double anchor system It has a static anchor composed of pins in titanium for stabilization of the cage in the intervertebral space, it also has a second anchor by means of screws towards … [Read more...] about ONIX
Paramount® Anterior Cervical Cage
The Paramount® Anterior Cervical Cage is a roughened, titanium anterior implant with integrated fixation plates. Paramount® Anterior Cervical Cage VIDEO ANIMATION Features: Controlled Anchor Plate Deployment The system has a threaded deployment mechanism to avoid aggressive impaction in the cervical spine. The integrated anchor plates are designed to deploy away … [Read more...] about Paramount® Anterior Cervical Cage
Pegasus Anchored Cervical Interbody
Pegasus Anchored Cervical Interbody (ACI) System provides surgeons a simplified approach to traditional ACDF. It features a single step delivery of a spacer with an integrated anchoring mechanism. Benefits: Surgeon controlled anchor deployment enables confidence Single-step, non-impaction implantation and locking mechanism, reduces operative time Simple intra-operative … [Read more...] about Pegasus Anchored Cervical Interbody
Pallas Low Profile Anterior Cervical Cage
Stand alone Implant for use in Cervical interbody fusion, which combines the functionality of a cervical interbody spacer with the benefits of an anterior Cervical Plate. Pallas Low Profile Anterior Cervical Cage.pdf Features: PEEK Interbody Space Radiopaque marker for posterior visualization during markingSpacer component is made of pure medical grade … [Read more...] about Pallas Low Profile Anterior Cervical Cage
PRO-LINK Ti Titanium Stand-Alone Cervical Spacer System
The PRO-LINK Stand-Alone cervical spacers are designed with a large, open graft area for maximum bone graft capacity. The spacers allow fusion at adjacent segments without the removal of an existing anterior cervical plate. The low-profile locking plate provides security against screw backout without disturbing surrounding soft tissue. About Life Spine Life Spine, … [Read more...] about PRO-LINK Ti Titanium Stand-Alone Cervical Spacer System
PRORAY™
Prodorth Cervical PEEK cage with screw provides a more reliable attachment to the vertebras due to the screw on it. The system offers an interbody fusion device with screw fixation and is intended to be used in ACDF procedures. Prodorth PEEK cage with titanium screws and locking mechanism provides a stable fixation without the need of an anterior … [Read more...] about PRORAY™