Spinal Simplicity announced that its IntraLink technology has been accepted into the FDA's Total Product Life Cycle Advisory Program (TAP), following its recent Breakthrough Device Designation. This milestone enhances collaboration with FDA experts and stakeholders to streamline regulatory strategy, address adoption barriers, and accelerate patient access. OVERLAND PARK, … [Read more...] about Spinal Simplicity’s IntraLink® Enrolled in FDA’s Total Product Life Cycle Advisory Program (TAP)
2025
IMPLANET Signs Exclusive Distribution Agreement with TINAVI Medical Technologies for the TiRobot® Spine Surgery System
Bordeaux, Boston, September 23, 2025 – 6:00 pm CEST - IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME), a medical technology company specializing in implants for orthopedic surgery and the distribution of advanced medical equipment, today announced the acceleration of its commercialization strategy for cobotic solutions in orthopedic surgery with the … [Read more...] about IMPLANET Signs Exclusive Distribution Agreement with TINAVI Medical Technologies for the TiRobot® Spine Surgery System
Carlsmed, Inc. Selected for Inclusion in Russell 2000® Index
CARLSBAD, Calif., Sept. 22, 2025 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), a medical technology company pioneering AI-enabled personalized spine surgery solutions, today announced that effective after market close on September 22, 2025, it has been added to the Russell 2000® Index. This was part of the planned additions of select … [Read more...] about Carlsmed, Inc. Selected for Inclusion in Russell 2000® Index
SpineGuard obtains European patent on automatic bone breach detection by DSG, applicable to orthopedic power drills and robots
PARIS and BOULDER (CO), September 22, 2025 – 8:30 am CEST - SpineGuard(FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced that its patent application directed to protect algorithms for automatic detection … [Read more...] about SpineGuard obtains European patent on automatic bone breach detection by DSG, applicable to orthopedic power drills and robots
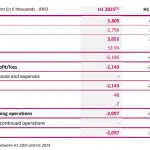
IMPLANET Announces First-Half 2025 Results
Bordeaux, Boston, September, 2025 – 5:45 p.m. CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME), a medical technology company specializing in implants for orthopedic surgery and the distribution of advanced medical equipment, today announced its results for the first half of the fiscal year ended June 30, 2025, as approved by the Board of Directors on … [Read more...] about IMPLANET Announces First-Half 2025 Results
Brainlab Announces FDA 510(k) Clearance and US Launch of Spine Mixed Reality Navigation
CHICAGO--(BUSINESS WIRE)--Brainlab, a digital medical technology company, has announced United States Food and Drug Administration (FDA) 510(k) clearance and US launch for Spine Mixed Reality Navigation. This advanced surgical navigation solution equips users with mixed reality support, delivering an ergonomic view of the navigation screen and allowing surgeons to place pedicle … [Read more...] about Brainlab Announces FDA 510(k) Clearance and US Launch of Spine Mixed Reality Navigation
Ruthless Spine Announces Intellectual Property News and 510(k) Clearance on Revolutionary Spinal Instrument
Device is designed to work in tandem with Ruthless Spine's De Novo cleared RJB™ intraoperative angle measurement instrument LOS ANGELES--(BUSINESS WIRE)-- Ruthless Spine today announced that it has received both U.S. Food and Drug Administration (FDA) 510(k) clearance and a U.S. patent for the NavJam™ Jamshidi, a new addition to its streamlined spinal platform. The device, … [Read more...] about Ruthless Spine Announces Intellectual Property News and 510(k) Clearance on Revolutionary Spinal Instrument
Aurora Spine Announces Launch of its DEXA-L™ Anterior Lumbar Interbody Fusion Device
CARLSBAD, Calif., Sept. 17, 2025 (GLOBE NEWSWIRE) -- Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a leader in innovative spine and interventional pain management solutions, today announces the official launch of its DEXA-L™ Anterior Lumbar Interbody Fusion Device, with the product being implanted in recent initial surgeries. DEXA-L is … [Read more...] about Aurora Spine Announces Launch of its DEXA-L™ Anterior Lumbar Interbody Fusion Device
Highridge Medical Enters Exclusive Distribution Agreement for the PathKeeper Surgical Navigation System
WESTMINSTER, Colo., Sept. 16, 2025 (GLOBE NEWSWIRE) -- Highridge Medical, one of the world’s largest privately held spine companies with an innovative and clinically supported portfolio, announced today it has entered into an exclusive distribution agreement for the PathKeeper Surgical Navigation System in the United States. The strategic agreement enhances Highridge’s enabling … [Read more...] about Highridge Medical Enters Exclusive Distribution Agreement for the PathKeeper Surgical Navigation System
Dallas County Judge Awards Medical Equipment Company $2.4M in Financial Fraud Case
DALLAS--(BUSINESS WIRE)--A Dallas County district court judge has awarded a Texas-based surgical implant provider $2.4 million in a financial fraud case involving a North Texas physician accused of stealing the company’s assets for his own enrichment. Beginning in 2020, orthopedic spine surgeon Dr. Venkat Sethuraman utilized biologics and hardware from USA Surgical and USA … [Read more...] about Dallas County Judge Awards Medical Equipment Company $2.4M in Financial Fraud Case
History-Making Verdict: Dismissal of All Anti-Kickback Charges Against CEO and Spine Surgeon Dr. Kingsley R. Chin
After seven years under DOJ investigation, all original Anti-Kickback charges were dismissed while Dr. Chin agreed to a plea settlement on a single Open Payments reporting issue and now moves forward to advance disruptive spine surgery innovations worldwide. FORT LAUDERDALE, Fla., Sept. 12, 2025 /PRNewswire/ -- Dr. Kingsley R. Chin today issued the … [Read more...] about History-Making Verdict: Dismissal of All Anti-Kickback Charges Against CEO and Spine Surgeon Dr. Kingsley R. Chin
B. Braun Acquires True Digital Surgery
GOLETA, Calif., Sept. 12, 2025 /PRNewswire/ -- B. Braun SE, a leading medical technology company, today announced the full acquisition of True Digital Surgery (TDS), a company based in Goleta, California, specializing in digital robotic-assisted 3D surgical microscopy. This acquisition highlights B. Braun's commitment to investing in the future of digital … [Read more...] about B. Braun Acquires True Digital Surgery
A new chapter begins at Tsunami Medical!
We are excited to welcome Marco Pozzi as our new Chief Commercial Officer (CCO). Marco’s career has been a journey through the spine industry: from product specialist to global marketing leader, to business development at an international level. Along the way, he has built strong relationships, driven innovation, and inspired growth. His story is one of passion, dedication, and … [Read more...] about A new chapter begins at Tsunami Medical!
Centinel Spine® Receives MDR Certification for prodisc® C Vivo and prodisc® C Nova Cervical Total Disc Replacement Systems
WEST CHESTER, Pa., Sept. 11, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced EU Medical Device Regulation … [Read more...] about Centinel Spine® Receives MDR Certification for prodisc® C Vivo and prodisc® C Nova Cervical Total Disc Replacement Systems
Nexus Spine’s Patented Tranquil® Interbody Implants Achieve Exceptional Outcomes
DRAPER, Utah, Sept. 11, 2025 /PRNewswire/ -- Nexus Spine is excited to share pilot study results of a comparative radiographic assessment of ACDF outcomes in a series of patients who were selected for common risk factors of subsidence, instability, and delayed bone growth. Specifically, the patients were over 66 years of age, had poor bone quality, and/or required … [Read more...] about Nexus Spine’s Patented Tranquil® Interbody Implants Achieve Exceptional Outcomes
Introducing EXALTA: A Bold New Brand Unifying Intech, Tyber and Resolve to Accelerate the Future of MedTech
One name. One team. One ambition. A new chapter to accelerate MedTech innovation. NEW YORK, NY, UNITED STATES, September 10, 2025 /EINPresswire.com/ -- Intech, Tyber Medical, and Resolve Surgical Technologies are proud to announce their rebrand under a unified identity: EXALTA. This transformation brings together three industry pioneers under one banner, reinforcing a shared … [Read more...] about Introducing EXALTA: A Bold New Brand Unifying Intech, Tyber and Resolve to Accelerate the Future of MedTech
OrthoPediatrics Corp. Completes First Procedures with VerteGlide™ Spinal Growth Guidance System
WARSAW, Ind., Sept. 10, 2025 (GLOBE NEWSWIRE) -- OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced the completion of the first U.S. surgical procedures with the VerteGlide Spinal Growth Guidance System (“VerteGlide”), used to treat Early Onset Scoliosis … [Read more...] about OrthoPediatrics Corp. Completes First Procedures with VerteGlide™ Spinal Growth Guidance System
Foundation Surgical Announces First-Ever Surgery Using the Interwedge®Standalone Lateral Implant System
Reading, PA – September 5, 2025 – Foundation Surgical today announced the successful completion of the first-ever surgical case using its newly FDA-cleared Interwedge® Standalone Lateral Implant System. The procedure was performed by Dr. Edward DelSole at Reading Hospital in Reading, Pennsylvania, is part of theTower Health healthcare system.The Interwedge® Standalone Lateral … [Read more...] about Foundation Surgical Announces First-Ever Surgery Using the Interwedge®Standalone Lateral Implant System
VB Spine Joins the American Spine Registry as Industry Sponsor
ROSEMONT, Ill., Sept. 5, 2025 /PRNewswire/ -- The American Spine Registry (ASR), a collaborative effort between the American Association of Neurological Surgeons (AANS) and the American Academy of Orthopaedic Surgeons (AAOS), today announced that VB Spine LLC (VB Spine) has joined as an official industry sponsor. This partnership advances the future of spine surgery through … [Read more...] about VB Spine Joins the American Spine Registry as Industry Sponsor