PLANO, Texas, May 22, 2025 /PRNewswire/ -- ulrich medical USA, a leader in spinal implant technologies and a subsidiary of the ulrich medical Group based in Ulm, Germany, is pleased to announce the appointment of Eric Gibbs as President and General Manager. Gibbs brings more than 25 years of leadership experience across sales, operations, and commercial strategy. His career … [Read more...] about ulrich medical USA® Appoints Eric Gibbs as President and General Manager
2025
After Diabetes, Could Medtronic’s Spine Division Be Its Next Spin-Off?
Medtronic’s announcement to spin off its diabetes business into a separate, publicly traded company surprised many. After all, diabetes is one of the fastest-growing global health challenges, and the technology supporting it — from smart insulin pumps to continuous glucose monitors — is advancing rapidly.But as the dust settles, the logic becomes clear: focus, operational … [Read more...] about After Diabetes, Could Medtronic’s Spine Division Be Its Next Spin-Off?
The Crown Is Still Medtronic’s… For Now!
A few months ago, we received numerous inquiries from our readers asking whether Globus Medical had finally surpassed Medtronic as the global leader in spinal technologies. Rather than speculate, we opted to wait for a clean, data-driven comparison between both companies’ most recent results. Although Medtronic operates on a fiscal calendar and Globus reports on a standard one, … [Read more...] about The Crown Is Still Medtronic’s… For Now!
Swiss Medtech and AdvaMed Call for Zero for Zero Tariffs and Recognition of FDA Approvals and Clearances
Bern/Washington (AdvaMed)– Swiss Medtech, the leading trade association for medical technology companies in Switzerland, and AdvaMed, the U.S. Medtech Association and the world’s largest organization representing medtech innovators, are jointly calling for reciprocal “zero for zero” tariffs between the two countries. Additionally, they advocate for Switzerland to become the … [Read more...] about Swiss Medtech and AdvaMed Call for Zero for Zero Tariffs and Recognition of FDA Approvals and Clearances
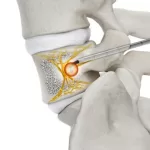
Nexxt Spine Launches New NEXXT MATRIXX® SI Sacroiliac Joint Fusion System
NOBLESVILLE, IN, UNITED STATES, May 21, 2025 /EINPresswire.com/ -- Nexxt Spine, LLC is proud to announce the launch of the NEXXT MATRIXX® SI System. The NEXXT MATRIXX SI System leverages the 3D-printed manufacturing sophistication of the NEXXT MATRIXX 3D printed technology, adding this new complementary offering for SI joint fusion procedures. The variety of implant options … [Read more...] about Nexxt Spine Launches New NEXXT MATRIXX® SI Sacroiliac Joint Fusion System
Medtronic reports strong finish to its fiscal year with its fourth quarter financial results; announces dividend increase
GALWAY, Ireland, May 21, 2025 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for its fourth quarter (Q4) and fiscal year 2025 (FY25), which ended April 25, 2025. Q4 Key Highlights Additional Key Highlights Q4 Financial ResultsMedtronic reported Q4 worldwide revenue … [Read more...] about Medtronic reports strong finish to its fiscal year with its fourth quarter financial results; announces dividend increase
Stryker receives FDA clearance for OptaBlate® BVN Basivertebral Nerve Ablation System
PORTAGE, Mich., May 19, 2025 /PRNewswire/ -- Stryker (NYSE:SYK), a global leader in medical technologies, announced that its OptaBlate basivertebral nerve ablation system (OptaBlate BVN) received 510(k) clearance from the U.S. Food and Drug Administration. OptaBlate BVNA is used in a targeted minimally invasive procedure providing long-lasting† vertebrogenic pain relief1. The … [Read more...] about Stryker receives FDA clearance for OptaBlate® BVN Basivertebral Nerve Ablation System
Globus Medical to Execute $500 Million Share Buyback Amid Market Undervaluation
May 16, 2025—Globus Medical (NYSE: GMED) announced yesterday the authorization of a $500 million share repurchase program. The company aims to capitalize on what it perceives as a disconnect between its market valuation and intrinsic value. The decision, disclosed via press release on May 15, reflects the company’s assessment of current market conditions and financial … [Read more...] about Globus Medical to Execute $500 Million Share Buyback Amid Market Undervaluation
A New Player in Spinal Care, POWEHI Medical!
POWEHI Medical is a German-based medical technology company dedicated to revolutionizing spinal surgery through cutting-edge, modular implant systems. Recognized among Germany’s most innovative mid-sized companies by STERN in 2023 and recipient of the Best Spine Technology Award at the 2022 NASS Conference in Chicago, POWEHI is setting new standards in spinal fixation. … [Read more...] about A New Player in Spinal Care, POWEHI Medical!
Jim Buck of Medcura, Inc. named EY US Entrepreneur Of The Year 2025 Mid-Atlantic Award Finalist
COLLEGE PARK, Md., May 15, 2025 /PRNewswire/ -- Medcura, Inc. today announced that President, CEO & Director Jim Buck, MBA is an Ernst & Young LLP (EY US) finalist for the prestigious Entrepreneur Of The Year 2025 Mid-Atlantic Award. Now in its 40th year, the Entrepreneur Of The Year program honors the bold leaders who disrupt markets through the world's most … [Read more...] about Jim Buck of Medcura, Inc. named EY US Entrepreneur Of The Year 2025 Mid-Atlantic Award Finalist
Genesys Spine Launches Stasys-C™ 3DP Cervical Stand-alone System
AUSTIN, Texas, May 14, 2025 /PRNewswire/ -- Genesys Spine is proud to announce the commercial launch of the Stasys-C™ 3DP Cervical Standalone System, a 3D-printed, zero-profile cervical interbody solution designed to simplify anterior cervical procedures while promoting early fusion. Engineered with a non-screw-based fixation method and inserted through a direct … [Read more...] about Genesys Spine Launches Stasys-C™ 3DP Cervical Stand-alone System
Xtant Medical Reports First Quarter 2025 Financial Results
BELGRADE, Mont., May 12, 2025 /PRNewswire/ -- Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal, orthopedic, and wound care disorders, today reported financial and operating results for the first quarter ended March 31, 2025. First Quarter 2025 Financial … [Read more...] about Xtant Medical Reports First Quarter 2025 Financial Results
SAFE Group – Successful first surgeries in Texas following purchase contract
Fleurieux-sur-l’Arbresle - France – May 12, 2025, 6.00 p.m. Safe Group (FR001400F1V2 – ALSAF), a leader in innovative medical solutions, is proud to announce the successful completion of the first surgeries at a new facility in Texas, where Safe Orthopaedics and Spine Up solutions are newly referenced (see press release of March 12, 2025). These operations mark an important … [Read more...] about SAFE Group – Successful first surgeries in Texas following purchase contract
Just Reflective, Not Disappointed: Globus Medical’s Bittersweet Q1 2025
Last week, Globus Medical reported its Q1 2025 results, and truth be told, many of us in the spine market were left with mixed feelings. Not disappointment, exactly, but perhaps a sense that expectations, particularly those fueled by previous quarters, weren’t fully met this time. Since the acquisition of NuVasive, Globus has made no secret of its ambition to catch and … [Read more...] about Just Reflective, Not Disappointed: Globus Medical’s Bittersweet Q1 2025
What’s Happening with Globus Medical? Why Has the Stock Dropped So Much? Will It Recover?
Globus Medical (NYSE: GMED) has experienced a significant drop in its stock price today, trading at $58.63 — a 19% decline today. Why Has It Dropped So Much? The drop in Globus Medical’s stock is due to several factors: Will It Recover? Despite these challenges, there are positive signs: Final Thoughts There’s no doubt that Globus Medical is facing a … [Read more...] about What’s Happening with Globus Medical? Why Has the Stock Dropped So Much? Will It Recover?
Osteotec Signs Exclusive Distribution Agreement with icotec ag to advance Spine Tumour Therapy in Great Britain
NEWBURY, England, May 9, 2025 /PRNewswire/ -- Osteotec, the market leading manufacturer and distributor of specialised medical devices, is pleased to announce an exclusive distribution agreement with icotec, the world leader of non-metallic spinal implants optimized for spine tumour therapy. This collaboration, effective from May 8, marks a key milestone in … [Read more...] about Osteotec Signs Exclusive Distribution Agreement with icotec ag to advance Spine Tumour Therapy in Great Britain
Globus Medical Reports First Quarter 2025 Results
AUDUBON, Pa., May 08, 2025 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the quarter ended March 31, 2025. “Our first quarter results were impacted by softer Enabling Technology deal closures, temporary integration related supply chain disruption, and timing of … [Read more...] about Globus Medical Reports First Quarter 2025 Results
Eminent Spine’s 3D Printed Titanium Pedicle Screw System Receives Groundbreaking FDA 510(k) Clearance. The Future of Fusion Has Arrived.
Plano, TX, May 07, 2025 --(PR.com)-- Eminent Spine, a leader in spinal implant innovation, proudly announces that its revolutionary 3D Printed Titanium Pedicle Screw System has received FDA 510(k) clearance as of April 28, 2025 — making it the first and only FDA 510(k)-cleared 3D printed pedicle screw system in the world. “This historic achievement represents a monumental … [Read more...] about Eminent Spine’s 3D Printed Titanium Pedicle Screw System Receives Groundbreaking FDA 510(k) Clearance. The Future of Fusion Has Arrived.
Guided Spinal Navigation Landmark Surgery Opens the Door to Cost-Effective 3D Augmented Reality
Salt Lake City, May 06, 2025 (GLOBE NEWSWIRE) -- A groundbreaking surgical procedure was successfully performed on a small island in the Mediterranean, marking a significant advancement in spinal surgery. At Hospital Son Llàtzer in Mallorca, Spain, Dr. Juan Toribio, a pioneer in orthopedic surgery, collaborated with Dr. Wendell Gibby, co-inventor of VisAR augmented reality … [Read more...] about Guided Spinal Navigation Landmark Surgery Opens the Door to Cost-Effective 3D Augmented Reality