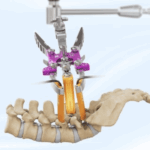
Three years ago, we examined the emerging role of 3D printing in medical devices, specifically focusing on interbody cages. By then, about 20 different 3D ALIF (Anterior Lumbar Interbody Fusion) cages were available. Today, just a few years later, that number has more than doubled, signaling rapid growth in both adoption and innovation.This upward trend highlights the … [Read more...] about (UPDATED 2024): +40 ALIF 3D Cages to Know!
2024
Intrinsic Therapeutics Announces New Category 1 CPT Code for Bone Anchored Annular Closure, including the Barricaid® Annular Closure Device, to Treat Patients Undergoing Lumbar Discectomy for Herniated Discs
BOSTON, Oct. 24, 2024 /PRNewswire/ -- Intrinsic Therapeutics, Inc., a medical device company focused on preventing reherniation and reoperation following lumbar discectomy to treat herniated discs, announced today that the American Medical Association (AMA) CPT Editorial Panel has accepted the addition of a new Category 1 CPT Code for bone anchored annular … [Read more...] about Intrinsic Therapeutics Announces New Category 1 CPT Code for Bone Anchored Annular Closure, including the Barricaid® Annular Closure Device, to Treat Patients Undergoing Lumbar Discectomy for Herniated Discs
Bone Biologics Appoints Phillip T. Meikle to its Board of Directors
BURLINGTON, Mass.--(BUSINESS WIRE)--Bone Biologics Corporation (“Bone Biologics” or the “Company”) (Nasdaq: BBLG, BBLGW), a developer of orthobiologic products for spine fusion markets, announces the appointment of Phillip T. Meikle to the Company’s board of directors, effective immediately. Mr. Meikle succeeds Don R. Hankey, who retired from the Board following seven years of … [Read more...] about Bone Biologics Appoints Phillip T. Meikle to its Board of Directors
(UPDATED 2024): +15 Midline Cervical Plates to Know!
Cervical plates have become less commonly utilized since the advent of stand-alone cervical cages. Historically, a variety of designs emerged, each aiming to address specific clinical needs. This evolution gave rise to dynamic plates, semi-rigid options, and neutral plates, including the midline variant. While midline plates have never gained significant popularity, they … [Read more...] about (UPDATED 2024): +15 Midline Cervical Plates to Know!
Fiagon GmbH Receives FDA Clearance for Spine Platform and Showcases Innovative Solutions at NASS and EUROSPINE 2024
BERLIN, GERMANY, October 17, 2024 /EINPresswire.com/ -- Fiagon GmbH is proud to announce a series of significant achievements, including the recent FDA clearance of the expanded Spine Platform and commercial launch in the United States at NASS 2024. Receiving FDA clearance for an extended platform from its Spine Navigation System Fiagon set a crucial milestone in the … [Read more...] about Fiagon GmbH Receives FDA Clearance for Spine Platform and Showcases Innovative Solutions at NASS and EUROSPINE 2024
Centinel Spine® Announces Record Revenue in Third Quarter 2024 as prodisc® Total Disc Replacement Growth Accelerates
WEST CHESTER, Pa., Oct. 17, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced achievement of record revenue in the third … [Read more...] about Centinel Spine® Announces Record Revenue in Third Quarter 2024 as prodisc® Total Disc Replacement Growth Accelerates
Life Spine Announces Continued Success and Industry Adoption of ProLift® Micro Endoscopic Expandable Spacer System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, Inc., a leading medical device company specializing in the design, development, manufacturing, and marketing of products for the surgical treatment of spinal disorders, today announced the ongoing adoption of its ProLift Micro Endoscopic Expandable Spacer System. This system utilizes the same Micro Invasive™ tube for access, disc … [Read more...] about Life Spine Announces Continued Success and Industry Adoption of ProLift® Micro Endoscopic Expandable Spacer System
4WEB Medical Names Brad Niemann as President
FRISCO, Texas, Oct. 16, 2024 /PRNewswire/ -- 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced today the appointment of Brad Niemann as President. "4WEB is excited to have Brad join the team and lead our organization through our next phase of … [Read more...] about 4WEB Medical Names Brad Niemann as President
Aurora Spine Corporation Announces Release of its Hydra Osteo Onyx Lumbar System
CARLSBAD, Calif., Oct. 15, 2024 (GLOBE NEWSWIRE) -- Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has launched its new OSTEO ONYX™ lumbar fusion system, promoting Rough Surface Technology (RST). “We are excited to … [Read more...] about Aurora Spine Corporation Announces Release of its Hydra Osteo Onyx Lumbar System
Oxford Performance Materials Customer Vy Spine Receives FDA Clearance for New OsteoFab® Lumbar Cage
SOUTH WINDSOR, Conn., Oct. 14, 2024 /PRNewswire-PRWeb/ -- Oxford Performance Materials, Inc. (OPM; https://oxfordpm.com/), an industry leader in advanced materials science and 3D printing solutions for the orthopedic industry, is pleased to announce that OPM customer Vy Spine (https://Vy Spine.com/), has received FDA clearance for the OsteoFab® Lumbar Cage, which follows Vy … [Read more...] about Oxford Performance Materials Customer Vy Spine Receives FDA Clearance for New OsteoFab® Lumbar Cage
SpineGuard Announces Its Q3 2024 Revenue
PARIS (France), BOULDER (CO, USA), October 9, 2024 – 6:00 pm CEST - SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced its third quarter 2024 revenue. Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, … [Read more...] about SpineGuard Announces Its Q3 2024 Revenue
The “Top Gun” Mentality in Spine Surgery!
In a recent article published on LinkedIn by Axis Technologies, a key topic is addressed regarding the future of spine surgery: the "Top Gun" mentality. Like elite pilots, spine surgeons face extraordinary challenges in an environment where millimeters are crucial and margins for error are non-existent. This parallel is not just a striking analogy, but a true representation of … [Read more...] about The “Top Gun” Mentality in Spine Surgery!
TeDan Surgical Innovations Launches Phantom ML3™ MIS Lumbar Surgical Access System, Offering Simplicity Without Compromising Versatility in Minimally Invasive TLIF Procedures
SUGAR LAND, Texas, Oct. 9, 2024 /PRNewswire/ -- TeDan Surgical Innovations, Inc. (TSI), a global leader in surgical access systems specializing in spine, neuro, orthopedic, and cardiothoracic surgery, has announced the launch of the Phantom ML3™ MIS Lumbar Surgical Access System. This innovative solution provides a simple, reproducible workflow for various … [Read more...] about TeDan Surgical Innovations Launches Phantom ML3™ MIS Lumbar Surgical Access System, Offering Simplicity Without Compromising Versatility in Minimally Invasive TLIF Procedures
Vy Spine Announces FDA Clearance of 3D Printed LumiVy OsteoVy PEKK Lumbar IBF
BOUNTIFUL, Utah--(BUSINESS WIRE)--Vy Spine, a spine innovation leader using differentiated materials and designs, announced today that it has received U.S. Food and Drug Administration (FDA) clearance for its LumiVy OsteoVy PEKK Lumbar IBF. The device is indicated for intervertebral body fusion for use at either one level or two contiguous levels in the lumbar spine, from L2 … [Read more...] about Vy Spine Announces FDA Clearance of 3D Printed LumiVy OsteoVy PEKK Lumbar IBF
Carlsmed Adds Industry Veteran, Kevin O’Boyle, to Board of Director
CARLSBAD, Calif.--(BUSINESS WIRE)--Carlsmed, the leader in personalized spine surgery, announced that Kevin O’Boyle has joined its Board of Directors and will serve as the Chair of the Audit Committee. Mr. O’Boyle previously served as a director of seven publicly traded companies, including Wright Medical Group (WMGI), GenMark Diagnostics (GNMK) and Zeltiq (ZLTQ). In … [Read more...] about Carlsmed Adds Industry Veteran, Kevin O’Boyle, to Board of Director
RTI Surgical Announces Agreement to Acquire Collagen Solutions
ALACHUA, Fla., October 7, 2024, RTI Surgical ("RTI" or "the Company"), a leading CDMO pushing the boundaries of innovation and tissue engineering to meet patient needs in regenerative medicine, announced today that it has entered into an agreement to acquire Collagen Solutions, a premier global supplier of engineered medical-grade collagen and xenograft tissue with applications … [Read more...] about RTI Surgical Announces Agreement to Acquire Collagen Solutions
KIC Ventures Changing the Private Equity Paradigm: The HealthTech Investment Firm with the Most Majority-Owned Portfolio of Revenue-Generating Spine Companies
FORT LAUDERDALE, Fla., Oct. 7, 2024 /PRNewswire-PRWeb/ -- KIC Ventures is reshaping private equity in healthtech by maintaining majority ownership in the most number of revenue-generating spine companies in the world. Unlike traditional firms that diversify minority stakes across sectors, KIC Ventures exclusively focuses on spine surgery technologies, giving it … [Read more...] about KIC Ventures Changing the Private Equity Paradigm: The HealthTech Investment Firm with the Most Majority-Owned Portfolio of Revenue-Generating Spine Companies
Bioretec updates its product development strategy by accelerating the product development of RemeOs™ Spinal Interbody Cage
TAMPERE, Finland, Oct. 4, 2024 /PRNewswire/ -- Bioretec updates its product development strategy by accelerating the product development of the RemeOs™ Spinal Interbody Cage, an innovative medical device engineered from a multifunctional, MRI-compatible, absorbable hybrid composite based on the company's proprietary magnesium alloy. The decision to accelerate … [Read more...] about Bioretec updates its product development strategy by accelerating the product development of RemeOs™ Spinal Interbody Cage
Foundation Surgical Secures 510(k) Clearance for Innovative Lateral Interbody System
Scottsdale, AZ – October 7, 2024--Foundation Surgical, an early-stage spinal implant company, is excited to announce the US Food &Drug Administration (FDA) 510(k) clearance of its Interwedge® Standalone Lateral Interbody System. This latest product clearance, used in conjunction with the Foundation’s new LLIF180™ procedure, represents a significant innovation in lateral … [Read more...] about Foundation Surgical Secures 510(k) Clearance for Innovative Lateral Interbody System