PARIS (France) and BOULDER (CO, USA), January 10, 2025 – 6:00 pm CET – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today reports its full-year 2024 revenue.
Pierre Jérôme, Chairman, CEO and co-founder of SpineGuard, said: “After a particularly challenging 2023 due to the termination of two important commercial agreements, SpineGuard is back to growth in 2024 particularly in the United States, by far the largest market of our sector, where we achieved 20% growth over the full year. The slowdown of our global revenue in Q4, mainly due to the temporary lack of sales from China, affected our annual global growth but we have good momentum in Europe and the Middle East. The strengthening of our partnership with Omnia Medical that we announced earlier this week, represents a major step for the future of SpineGuard. It enables us to immediately bolster the US launch of PsiFGuard scheduled end of January at the NANS annual meeting in Orlando, Florida. The feedback received by the first physicians using this cannulated probe equipped with our DSG sensor to facilitate the insertion of sacroiliac implants, is extremely encouraging. This strategic alliance will allow us to optimize our strengths and assets as we implement, step by step, the road map agreed upon with Omnia.”
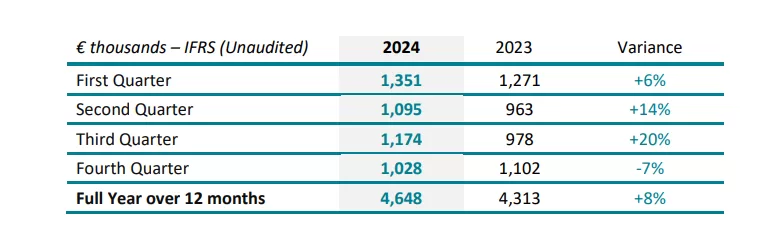
SpineGuard’s consolidated full year revenue increased by 8% in 2024 compared to 2023.
In the United States, the revenue increased by 20% in 2024 compared to 2023. This growth was mainly driven by significant orders from Omnia Medical, regained direct access to the pediatric accounts previously addressed by Wishbone Medical, and the positive impact of the new American team put in place by SpineGuard last spring.
Europe, mainly driven by Greece, Slovakia, Germany and The Netherlands, grew by 4%, and the Middle East by 19%. Latin America decreased by 12% and Asia by 64% due to the temporary lack of orders from XinRong Medical, SpineGuard’s distributor in China. The Chinese medical device market faced several upheavals in 2024 which have slowed down XinRong in its commercial efforts, however the situation has been improving in the last few months. The approval by the NMPA (National Medical Products Administration, China’s equivalent of the FDA) of the Curved and Miniaturized PediGuard products expected in H1 2025 will be an important milestone for the commercial deployment of the DSG technology in China, second largest market for spinal devices after the United States.
Over the fourth quarter 2024, salesincreased by 2% in the United States. Europe recorded a 15% growth, and the Middle East 284%. Latin America dropped by 22% and Asia by 87% due to a large Chinese order invoiced in the fourth quarter 2023. 6,142 DSG units were sold full year in 2024 vs. 6,138 units full year in 2023. 2,489 units were sold in the United States representing 41% of total units.
2025 Outlook
In 2025, SpineGuard will continue to focus on sustaining its sales momentum, by relying on the introduction of its new products on the market, the Threaded PediGuard for scoliosis correction via anterior approach recently CE marked, and the PsiFGuard device co-developed with Omnia Medical for sacroiliac fusion which recently obtained FDA clearance. The Company is also pursuing the registering process of the whole PediGuard product range in China. Moreover, SpineGuard is working on building strategic partnerships and on strengthening its financial position in continuing to explore various financing options. To date, the Board of Directors has adopted the going-concern principle based on the Company’s consolidated cash position, cash equivalents, forecast inflows, ongoing commercial growth, the recent signature of a bond contract for a maximum amount of 1 million euros and the measures implemented by the management to secure the Company’s financing.
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices embedding its technology. Over 100,000 surgical procedures have been secured worldwide thanks to DSG® and 34 studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard is expanding the scope of its DSG® technology to the treatment of scoliosis via anterior approach, sacroiliac joint fusion, dental implantology and innovations such as the « smart » pedicle screw and power drill or surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives. For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
SpineGuard
Pierre Jérôme
CEO & Chairman
Tel: +33 1 45 18 45 19
p.jerome@spineguard.com
SpineGuard
Manuel Lanfossi
CFO
Tel: +33 1 45 18 45 19
m.lanfossi@spineguard.com
NewCap
Investor Relations & Financial Communication
Mathilde Bohin / Aurélie Manavarere
Tel: +33 1 44 71 94 94
spineguard@newcap.eu
