PARIS (France), BOULDER (CO, USA), October 9, 2024 – 6:00 pm CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced its third quarter 2024 revenue.
Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, stated: “SpineGuard sales growth continued to accelerate in the third quarter 2024, up 20%, driven by the strengthened and now well-established US commercial structure and our partnership with Omnia Medical. The CE-MDR certification obtained early September for the utilization in Europe of the Threaded PediGuard for scoliosis correction via anterior approach and the clearance in the United States recently announced of the “PsiFGuard” device co-developed with Omnia Medical to secure the sacroiliac fusion, constitute two major regulatory steps that will enable us to sustain our commercial momentum moving forward. These two new DSG technology applications are indeed particularly promising given the market opportunities they represent. We already received extremely positive feedback from early adopters. All these advances confirm, once again, that we are on track to reach our goal of financial breakeven in the course of 2026.”
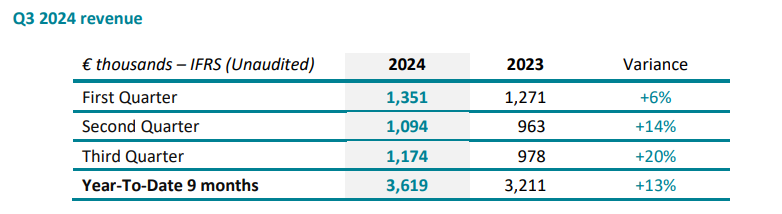
SpineGuard’s consolidated revenue sets at € 1,174K in Q3 2024, up 20% compared to Q3 2023, bringing revenue for the first nine months of 2024 to € 3,619K, up 13% over € 3,211K recorded for the corresponding period in 2023.
In the United States, the third quarter 2024 revenue is up 30% in USD, to $ 947K from $ 728K, mainly spurred by the recovery driven by the now well-established American team and a large order from Omnia Medical. In the rest of the world, the third quarter 2024 revenue grew by 1%.
4,813 DSG units were sold in the first nine months of 2024 vs. 4,395 units in the first nine months of 2023, representing an overall growth of 10% in units sold. 1,987 units were sold in the United States representing 41% of total units.
Q4 2024 outlook
In the fourth quarter of 2024, SpineGuard will continue to focus on sustaining its sales momentum, by relying on the introduction of its new products on the market, the Threaded PediGuard for scoliosis correction via anterior approach recently CE marked, and the PsiFGuard device co-developed with Omnia Medical for sacroiliac fusion which just obtained FDA clearance. The Company is also pursuing the registering process of the whole PediGuard product range in China. Moreover, SpineGuard is working on building new strategic partnerships and on strengthening its financial position.
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices embedding its technology. Over 100,000 surgical procedures have been secured worldwide thanks to DSG® and 34 studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard is expanding the scope of its DSG® technology to the treatment of scoliosis via anterior approach, sacroiliac joint fusion, dental implantology and innovations such as the « smart » pedicle screw and power drill or surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
SpineGuard
Pierre Jérôme
CEO & Chairman
Tel: +33 1 45 18 45 19
p.jerome@spineguard.com
SpineGuard
Anne-Charlotte Millard
CFO
Tel.: 01 45 18 45 19
ac.millard@spineguard.com
NewCap
Investor Relations & Financial Communication
Mathilde Bohin / Aurélie Manavarere
Tel: +33 1 44 71 94 94
spineguard@newcap.eu
