Global C-suite leader brings decades of spinal innovation and PMA experience to support Dymicron's next stage of growth OREM, Utah, Feb. 19, 2026 /PRNewswire/ -- Dymicron®, a privately held medical device company advancing next-generation spinal motion-preservation technology, today announced the appointment of Peter Wehrly, one … [Read More...] about Dymicron Appoints Veteran MedTech CEO Peter Wehrly to Board of Directors
Main Content
FEATURED NEWS
BREAKING NEWS

Carlsmed Announces first corra™ personalized Cervical Plating Procedure
The corra™ Cervical Plating System marks the debut of Carlsmed's patient-specific fixation portfolio CARLSBAD, Calif., Feb. 18, 2026 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), today announced the first personalized cervical plating procedure using the corra™ Cervical Plating System, the latest addition to its portfolio of personalized spine surgery … [Read More...] about Carlsmed Announces first corra™ personalized Cervical Plating Procedure
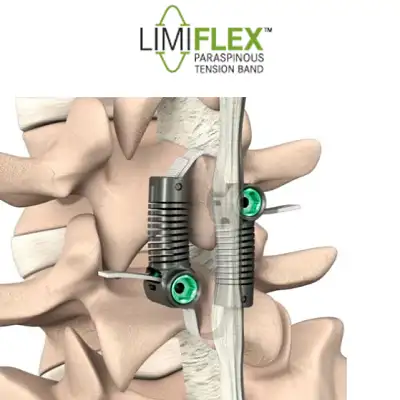
FDA Grants PMA Approval to LimiFlex(TM) Dynamic Sagittal Tether
Motion-Preserving Lumbar Spine Surgery - Stabilization Without Fusion for Patients with Degenerative Spondylolisthesis SAN CARLOS, Calif., February 18, 2026 (Newswire.com) - The U.S. Food and Drug Administration (FDA) has granted Premarket Approval (PMA) to the LimiFlex™ Dynamic Sagittal Tether, a motion-preserving system for the treatment of lumbar spinal stenosis associated … [Read More...] about FDA Grants PMA Approval to LimiFlex(TM) Dynamic Sagittal Tether

Spineway : 2025 annual results
Ecully, February 17, 2026, SPINEWAY 2025 annual results: * 2024 Pro forma: changes to the 2025 French General Chart of Accounts (ANC Regulation 2022-06) required the inclusion in operating income/(loss) of items that were previously recognized in exceptional income and expenses. Additionally, the Research and Innovation tax credits, which were recognized in operating income until 2024, are … [Read More...] about Spineway : 2025 annual results

Fziomed Commemorates One Millionth Patient Treated With Its Dual-Polymer Adhesion Barrier Technology
SAN LUIS OBISPO, Calif., Feb. 18, 2026 /PRNewswire/ -- Fziomed, Inc. ("Fziomed" or the "Company"), a recognized global leader in postsurgical adhesion prevention with the best-in-class synthetic, absorbable gel technology platform, today announced that the Company has surpassed treating one million patients worldwide across its product portfolio over the past 20+ … [Read More...] about Fziomed Commemorates One Millionth Patient Treated With Its Dual-Polymer Adhesion Barrier Technology

Medtronic reports strong third quarter fiscal 2026 results with highest enterprise revenue growth in 10 quarters
GALWAY, Ireland, Feb. 17, 2026 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for its third quarter (Q3) of fiscal year 2026 (FY26), which ended January 23, 2026. Q3 Key Highlights "Q3 marks another strong quarter, delivering 6% organic revenue growth, ahead of guidance, demonstrating the strength of our portfolio," … [Read More...] about Medtronic reports strong third quarter fiscal 2026 results with highest enterprise revenue growth in 10 quarters

Life Spine Announces MR Conditional Status for ProLift Expandable Spacer System Product Lines
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, a medical device company that designs, develops, manufactures, and markets products for the surgical treatment of spinal disorders, announced today its ProLift® expandable interbody portfolio has been evaluated and confirmed as MR Conditional in accordance with ASTM standards. The MR Conditional status includes ProLift, ProLift Micro, and ProLift … [Read More...] about Life Spine Announces MR Conditional Status for ProLift Expandable Spacer System Product Lines

Medical Sales Isn’t One Career. Which One Are You Actually In?
Many careers in medical sales fail not because of performance, but because people are hired into the wrong type of role. From the outside, healthcare commercial roles look similar. A badge, a hospital, meetings with clinicians, and a product to represent. But inside the industry, the term “sales rep” describes fundamentally different professions. A trauma rep, a capital equipment … [Read More...] about Medical Sales Isn’t One Career. Which One Are You Actually In?

We are proud to announce that Tsunami Medical will once again be a sponsor of SPINEMarketGroup in 2026
Thank you, Tsunami Medical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About Tsunami Medical The company has been founded in 1997 as a subcontractor of some big manufacturing companies of invasive diagnostic devices. Over the years we bought … [Read More...] about We are proud to announce that Tsunami Medical will once again be a sponsor of SPINEMarketGroup in 2026

VB Spine Announces Intent to Acquire Exclusive Rights to Augmedics’ Spine Platform
NEW YORK & CHICAGO--(BUSINESS WIRE)--VB Spine LLC (“VB Spine”), the largest privately held spine company, today announced it has entered into a definitive agreement to acquire exclusive rights to the xvision Spine System® (xvision) from Augmedics, adding augmented reality (AR) navigation to VB Spine’s growing enhanced visualization portfolio. Augmedics is the first company to offer an … [Read More...] about VB Spine Announces Intent to Acquire Exclusive Rights to Augmedics’ Spine Platform