Globus Medical is not simply entering 2026 with an “aggressive” posture. It is attempting something structurally more ambitious: building a self-reinforcing spine ecosystem that compresses competitive space around it. This is not a growth story. It is a control story. Spine as the Economic Engine Roughly 95% of revenue still comes from spine. … [Read More...] about Globus Medical’s Strategy: Building a Closed-Loop Spine Ecosystem
Main Content
FEATURED NEWS
BREAKING NEWS

SMAIO Announces a €3.6 Million Capital Increase to Support Its Long-Term Growth Momentum
DALLAS & LYON, France--(BUSINESS WIRE)--Regulatory News: This press release may not be published, distributed or disseminated, directly or indirectly, in the United States of America or Canada, Australia, Japan or South Africa. SMAIO (Software, Machines and Adaptive Implants in Orthopaedics – Euronext Growth Paris, ISIN: FR0014005I80 / Ticker: ALSMA, eligible for … [Read More...] about SMAIO Announces a €3.6 Million Capital Increase to Support Its Long-Term Growth Momentum

ulrich medical USA® Appoints Alissa Calaway, RN, MSN as Director of U.S. Clinical Affairs; Expands Commitment to Evidence-Based Spine Innovation
PLANO, Texas, March 5, 2026 /PRNewswire/ -- ulrich medical USA is pleased to announce the appointment of Alissa Calaway, RN, MSN, as Director of U.S. Clinical Affairs. Alongside this leadership appointment, the company is launching a U.S.-based Clinical Research Program designed to generate clinical evidence supporting product safety, performance, and patient outcomes … [Read More...] about ulrich medical USA® Appoints Alissa Calaway, RN, MSN as Director of U.S. Clinical Affairs; Expands Commitment to Evidence-Based Spine Innovation

Intrinsic Therapeutics Announces Initial Release of Its Next Generation Barricaid Narrow Anchor Device Designed to Reduce Reherniation After Back Surgery
BOSTON, March 4, 2026 /PRNewswire/ -- Intrinsic Therapeutics, Inc. has introduced the Barricaid® Narrow Anchor, the next generation of its FDA PMA-approved Barricaid® Bone-Anchored Annular Closure Device. The new version of the Barricaid device is 25 percent smaller than the standard Barricaid anchor. This updated device is designed to make implantation easier by reducing … [Read More...] about Intrinsic Therapeutics Announces Initial Release of Its Next Generation Barricaid Narrow Anchor Device Designed to Reduce Reherniation After Back Surgery

Xtant Medical Finalizes Companion Spine Transactions
BELGRADE, Mont., March 2, 2026 /PRNewswire/ -- Xtant Medical Holdings, Inc. (NYSE American: XTNT), a medical technology company focused on surgical solutions for spinal and other orthopedic conditions, today announced that it has received $10.7 million from Companion Spine related to Companion Spine's purchase of Xtant's Coflex® assets and Paradigm OUS businesses in December … [Read More...] about Xtant Medical Finalizes Companion Spine Transactions
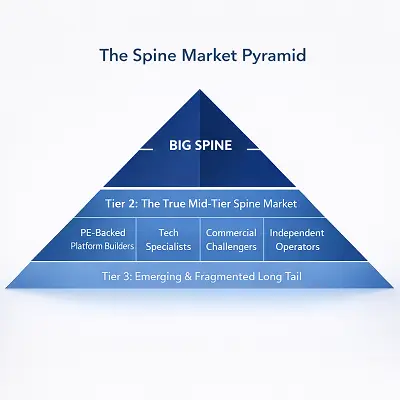
Who Is Really Winning the Mid-Tier Spine Consolidation?A Structural Map of the Competitive Landscape
The mid-tier spine market is not quietly evolving. It is being compressed, reshaped and selectively strengthened by structural forces that increasingly reward scale, differentiation and capital discipline. What often appears to be routine M&A activity is, in reality, a multi-year sorting process that is steadily separating structurally advantaged players from those simply trying to keep … [Read More...] about Who Is Really Winning the Mid-Tier Spine Consolidation?A Structural Map of the Competitive Landscape

CityUHK research team develops 3D-printed biomimetic “mechanoelectrical” smart materials inspired by sea urchin spines
HONG KONG, March 1, 2026 /PRNewswire/ -- A research team led by Professor Lu Jian, Dean of the College of Engineering and Chair Professor in the Department of Mechanical Engineering at City University of Hong Kong (CityUHK), has discovered that the naturally occurring porous ceramic structure within sea urchin spines possesses an unexpected capability for mechanoelectrical … [Read More...] about CityUHK research team develops 3D-printed biomimetic “mechanoelectrical” smart materials inspired by sea urchin spines

Synergy Spine Solutions® Receives FDA Approval for its Synergy Disc®, Expanding Cervical Disc Replacement Options for U.S. Patients
LOUISVILLE, Colo., Feb. 27, 2026 /PRNewswire/ -- Synergy Spine Solutions®, a medical device company focused on improving the quality of life for patients undergoing spine surgery, today announced it has received U.S. Food and Drug Administration (FDA) Premarket Approval (PMA) for the Synergy Disc® for 1-level indications at C3-C7. The Synergy Disc achieved superiority to the fusion control on the … [Read More...] about Synergy Spine Solutions® Receives FDA Approval for its Synergy Disc®, Expanding Cervical Disc Replacement Options for U.S. Patients

Highridge Medical Begins a Limited Launch of the activL® Lumbar Disc with Strong Early Surgeon Adoption
WESTMINSTER, Colo., Feb. 26, 2026 (GLOBE NEWSWIRE) -- Highridge Medical, a leading privately held global spine company with a portfolio supported by extensive clinical evidence, today announced the U.S. limited launch of the activL® Lumbar Disc, along with the successful completion of initial surgeries at multiple disc replacement centers nationwide. As part of the launch, Highridge Medical … [Read More...] about Highridge Medical Begins a Limited Launch of the activL® Lumbar Disc with Strong Early Surgeon Adoption

ZSFab Announces First Clinical Use of 20° Hyperlordotic Cervical Cages in Puerto Rico
WALTHAM, Mass., Feb. 26, 2026 (GLOBE NEWSWIRE) -- Dr. Fernando Villamil recently performed a three-level anterior cervical discectomy and fusion (ACDF) at Ashford Hospital in Puerto Rico using ZSFab’s 20° InterConnect™ hyperlordotic cervical cages. This case represents the first clinical use of our 20° hyperlordotic implant design, which incorporates advanced 3D-printed Triply Periodic Minimal … [Read More...] about ZSFab Announces First Clinical Use of 20° Hyperlordotic Cervical Cages in Puerto Rico

Expanding Innovations Launches Enabling Technologies Division in North Carolina’s Research Triangle
MOUNTAIN VIEW, Calif., Feb. 26, 2026 /PRNewswire/ -- Expanding Innovations today announced the launch of its Enabling Technologies Division, a new facility located in North Carolina's Research Triangle. This strategic investment will accelerate the company's development of a suite of intraoperative diagnostic tools designed to provide real-time assessment and protection of vertebral … [Read More...] about Expanding Innovations Launches Enabling Technologies Division in North Carolina’s Research Triangle

Behind the Deal: The Strategic Logic of Zavation’s ChoiceSpine Acquisition
Zavation Medical’s acquisition of ChoiceSpine looks like a strategically sound step within the ongoing consolidation of the spine mid-market. With private equity firm Gemspring Capital behind the deal, the rationale is familiar: bring together complementary platforms, expand commercial reach, improve EBITDA potential, and ultimately build a more valuable asset ahead of a future liquidity … [Read More...] about Behind the Deal: The Strategic Logic of Zavation’s ChoiceSpine Acquisition