The global market for smart implants is estimated to have a market value of more than $22.2 billion by 2032. Soon, many companies will create revolutionary implants with better outcomes and cost savings. A few weeks ago we talked about the company Intelligent Implants as an innovative company in the smart cages segment. Today we update this article by adding the company DirectSync Surgical that recently received Breakthrough Device Designation from FDA for its Patient-Powered Smart Implants.
Smart Implant Companies.
1.- Intelligent Implants
Intelligent Implants is a medical device company operating at the intersection of digital medicine and orthopedics. We develop innovative electrotherapeutic orthopedic devices to address significant unmet clinical needs. The company’s SmartFuse® platform is a wirelessly enabled, active implant technology that uses an array of electrodes to stimulate, control, and monitor bone growth. Our mission is to leverage SmartFuse to develop a pipeline of smart orthopedic implants that can reduce healing times, engage patients & surgeons, enable digital surgery/robotics and ultimately improve outcomes for millions of patients.
Website: http://www.intelligentimplants.co
Its first product, the SmartFuse® System, incorporates an electrotherapeutic technology platform into an intervertebral spacer (SmartFuse CAGE™) to improve outcomes in lumbar spinal fusion procedures. The implant uses electrical signals and an array of electrodes to accelerate and measure bone growth. The SmartFuse CLOUD™ can provide unprecedented visibility into healing with remote data to support real-time clinical decision-making.
What benefits do these implants provide (according to the manufacturer)?
- Accelerated Healing: Local delivery of electrical stimulation provides accelerated bone growth exactly where needed. The multi-electrode array controls the healing.
- Real-Time Data: Physician Portal and SmartFuse Cloud provide the surgeon with remote information on patient progress in real-time. It includes data on system function, patient compliance, and bone growth. The patient-facing App is aimed at enhancing usability and improving compliance.
- Minimally invasive: The microelectronic “guts” of the SmartFuse System were designed to be seamlessly incorporated into orthopedic implants and compatible with all existing surgical procedures including minimally invasive techniques. Additionally, wireless power and communication result in a fully contained implant and obviate the need for implanted batteries or indwelling leads.
How does it work? Please watch the VIDEO Animation: Intelligent Implants: SmartFuse CAGE™
The SmartFuse System:

- The SmartFuse Cage:The PEEK and titanium implant uses hermetically sealed electronics and an array of electrodes to accelerate, control, and measure bone growth. The CAGE was designed to be implanted with standard surgical procedures.
- The SmartFuse ECaP: The External Control and Power module (ECaP) is a cloud-connected medical wearable that wirelessly powers and communicates with the CAGE. The ECaP is the “control center” for the SmartFuse System; activating and controlling the CAGE while simultaneously collecting and transmitting data to the SmartFuse CLOUD. During use, the ECaP is designed to be comfortably worn by the patient in a corset which places the ECaP in close proximity to the CAGE to enable wireless control and power.
- The SmartFuse CLOUD:The SmartFuse CLOUD links the physician and the patient to the SmartFuse System via the Physician PORTAL and the Patient APP. The SmartFuse CLOUD enables the Physician to remotely monitor patient compliance, system status, and measurements of bone growth; thus providing important data to support real-time clinical decision making. The SmartFuse Patient APP is a wellness product that supports compliance coaching and tracking.
Status: (May 2021): Intelligent Implants Ltd announced that its SmartFuse® system had received Breakthrough Device Designation by the US Food and Drug Administration (FDA).
2.- DirectSync Surgical (formerly Evoke Medical)
DirectSync Surgical is pioneering a new generation of human powered smart implants that enhance the body’s natural ability to heal & provides unprecedented post-operative implant data to the physician and their patients. Website: https://www.directsyncsurg.com
Platform Technology
DirectSync Surgical has designed a fully integrated smart implant that senses the load and electrically stimulates bone growth at the primary site of implantation – no batteries or external stimulators are required!
Their proprietary technology mimics the natural healing properties of bone by producing a small pulse of DC electrical stimulation in sync with each step the patient takes. This core innovation has applications in multiple load-bearing orthopedic implants.
Key Accomplishments
- FDA Breakthrough Device Designation
- $1.75M in non-dilutive NIH SBIR funding
- 2 pre-clinical animal studies
- Issued foundational IP & other pending IP
- Completed FDA pre-submission meeting
How does it work? DirectSync Surgical VIDEO ANIMATION
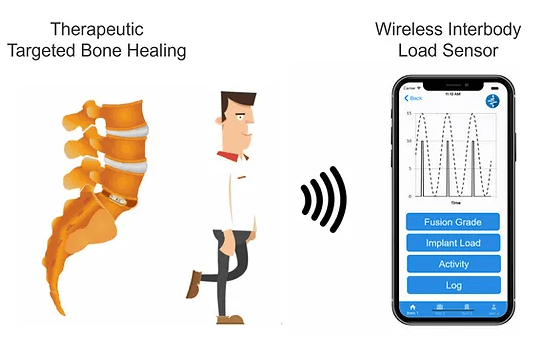
Status (May 2023) : DirectSync Surgical has successfully completed a NIH Phase II SBIR grant that supported a comparative randomized pre-clinical bone growth assessment and is currently completing a second NIH Phase I SBIR grant to integrate diagnostics to monitor key healing metrics. DirectSync Surgical is raising a Seed Round to accelerate efforts to initiate first-in-human studies.
####
All video parts, images, and documents related to the products are the sole property of the different companies. All the information is for Educational purposes only! No copyright infringement intended. We encourage you to contact us if you have any comments or suggestions or if you want us to include/remove your videos, images, or brochures. Please contact us at: spinemarketgroup@gmail.com
