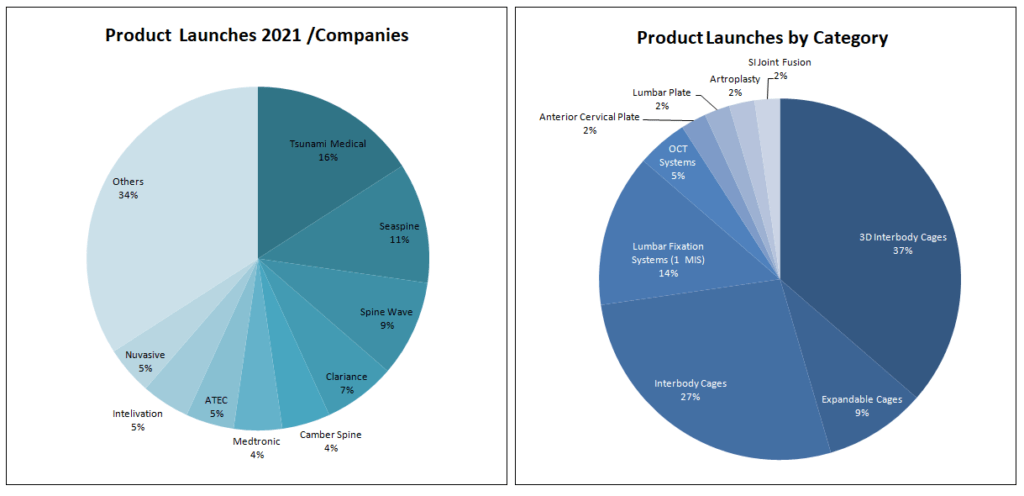
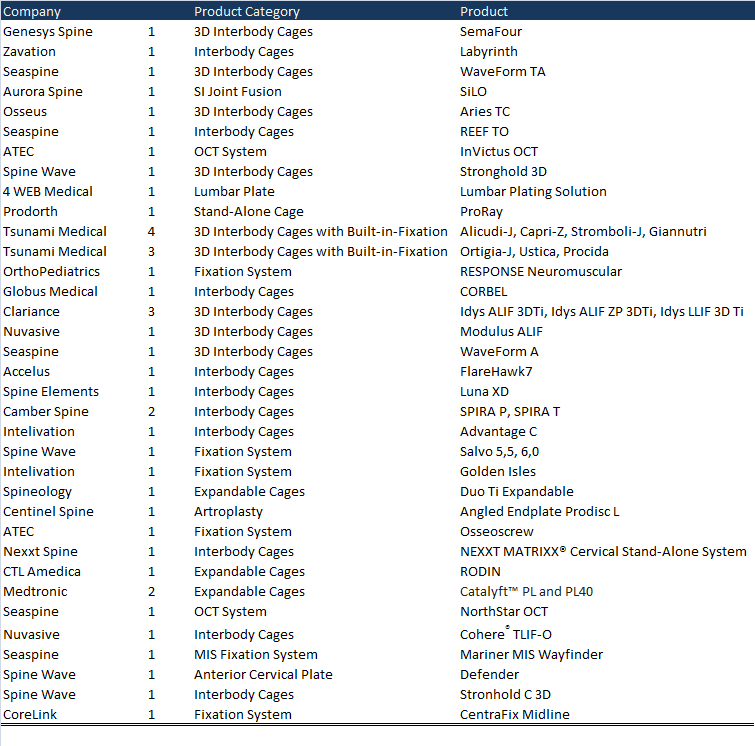
In 2021, at least 44 spine products were launched on the market. According to our information, the companies that have been most active in 2021, launching more than two new products have been: Tsunami Medical, Seaspine, Spine Wave, Clariance Spine, Camber Spine, Medtronic, ATEC, Intelivation and, Nuvasive. These companies represent 66% of all launches this year (Chart 1).
Noteworthy is the launch of a complete range of Expandable 3D cages by the Italian company Tsunami Medical, the Lumbar anterior cage releases by Clariance Spine and, the new 3D cages launched by Genesys Spine.
List of 44 product launches in 2021
35 Product Launches:

1.- 3DP Lumbar Interbody with SemaFour™ lattice technology | Genesys Spine
January 28th, 2001 Genesys Spine announced the launch of their new 3DP Lumbar Interbodies. They are additively manufactured from titanium alloy using a proprietary design.The science behind our proprietary SemaFour™ lattice technology was rigorously researched to provide the best possible osteogenic potential.
The SemaFour™ lattice technology is characterized by the optimization of four key facets: Surface Roughness, Surface Energy, Wettability, and Pore Size.These design elements were tailored specifically to aid the healing cascade and to promote the fusion process. End-users will also be thrilled by the low-modulus, highly porous, osteoconductive nature of our 3D-printed Interbodies. The ALIF portion of this system is currently available for use – all other approaches are available upon request.Geometric Optimization was developed to accelerate angiogenesis and osteogenesis through pore-size, porosity, pore-interconnectedness, and stiffness.
2.-LABYRINTH™ | Zavation
February 9, 2021, Zavation Medical Products , announced the launch of LABYRINTH™, an innovative porous PEEK interbody cage.LABYRINTH™, the latest addition to Zavation’s portfolio, features the first available porous endplates integrated through the full cage, setting the new gold standard for interbody cages. In creating the first fully porous PEEK interbody, Zavation has enhanced the benefits of PEEK (reduced stress shielding and artifact-free imaging) with substantially improved wettability and surface tension. In a 21 day in-vitro study, the interbody’s proprietary and patent pending porous structure demonstrated greater pre-osteoblast cell maturation into viable bone cells than standard PEEK, porous titanium, and standard titanium.
3.-WaveForm TA | Seaspine
February 22, 2021, SeaSpine announced the limited commercial launch of its 3D-printed WaveForm TA (TLIF Articulating) Interbody Implant System. WaveForm TA represents SeaSpine’s first 3D-printed lumbar interbody system and follows the September 2020 launch of its WaveForm C Interbody Implant System, designed to be used in ACDF (anterior cervical discectomy fusion) procedures. SeaSpine will be launching three additional 3D-printed interbody systems by mid-2021.
The WaveForm TA implant offers the next level of 3D-printed architectural innovation, balancing key geometric and manufacturing advancements without compromising clinical requirements. WaveForm TA utilizes innovative WaveForm technology to deliver a highly porous and robust interbody solution. WaveForm TA features 65% porous endplates* and 75% porous architecture within the body* of the implant. This design is intended to balance subsidence resistance, implant stiffness, and bone graft packability, while maintaining radiographic visualization during intraoperative and postoperative imaging.
WaveForm TA is designed to be used in TLIF (transforaminal lumbar interbody fusion) procedures. The system includes multiple footprints and lordotic options, allowing surgeons the ability to intraoperatively address specific anatomical needs. The 3D-printed interbody devices utilize the same instrumentation as the Reef™ TA Interbody System that SeaSpine launched in 2020. By deliberately designing best-in-class instruments to be utilized with two different surface technologies, such as WaveForm and Reef Topography™, SeaSpine is minimizing instruments required to be onsite at the hospital yet giving surgeons the options that they prefer.
4.-SiLOTM | Aurora Spine
March 01, 2021, Aurora Spine Corporation announced it has successfully launched its proprietary SiLOTM Posterior SI Joint Fusion System (SiLO), which was designed specifically for posterior sacroiliac joint fusion.
Sacroiliac joint (SI Joint) fusion is a surgical procedure which fuses the iliac bone (pelvis) to the spine (sacrum) for stabilization. The SiLOTM Posterior SI Joint Fusion System is a single graft Posterior SI-Fusion System made of human cortical bone and was developed to provide a simple, safe, and reproducible method of stabilizing and fusing the sacroiliac joint.
The SiLO graft was designed specifically for posterior sacroiliac joint fusions and consists of three levels of ridges along its circumferential solid body to increase implant retention and stability through its unique “Dowel Anchorage Design.” The SiLO graft is shaped for enhanced 360-degree bone incorporation and includes vertical side-channels that allow for additional bone graft material during insertion for enhanced stability. The SiLO graft is implanted with custom designed, patent pending instrumentation.
5.-Aries™-TC | Osseus Fusion Systems
March 1, 2021, Osseus Fusion Systems announced the official launch of Aries™-TC, its 3D printed transforaminal curved interbody fusion device.
Aries™ devices are constructed from highly porous, 3D printed titanium optimized for bone fusion and biological fixation using PL3XUS, Osseus’ proprietary 3D-printing technology. PL3XUS titanium technology utilizes powder bed fusion, specifically Selective Laser Melting (SLM), to create a three-dimensional diamond lattice network with roughened surface topography designed to promote bony fusion onto, into, and throughout the device. The Aries™-TC lumbar interbody fusion device comes in a wide variety of footprints, heights, and lordotic angles, to accommodate wide a variety of patient anatomies.
The system is intended for intervertebral body fusion procedures in skeletally mature patients with degenerative disc disease (DDD) of the lumbar spine at one or two contiguous levels from L2 to S1. It also offers aid to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s).
The next Osseus 3D printed interbody to hit the market will be the Pisces-SA Standalone ALIF Interbody System later this year.

6.-Reef TO (TLIF Oblique) Interbody System | SeaSpine
March 01, 2021 SeaSpine Holdings Corporation announced the full commercial launch of the Reef TO (TLIF Oblique) Interbody System.
The Reef TO Interbody System is designed for posterior lumbar interbody procedures and accommodates both direct impact insertion and insert-and-rotate techniques. With a comprehensive set of decompression, disc preparation, and implant insertion instruments, Reef TO provides a versatile and reproducible lumbar interbody solution for surgeons.
Reef TO interbody devices feature NanoMetalene® surface technology and Reef Topography™. NanoMetalene is a sub-micron layer of commercially pure titanium bonded to a PEEK implant, designed to provide a bone-friendly titanium surface, while retaining the benefits associated with traditional PEEK, such as biocompatibility, a modulus of elasticity similar to bone, and excellent radiographic visibility for post-operative imaging. The added macro structures of Reef Topography provide greater titanium surface area and improved biomechanical stability.
7.-InVictus OCT Spinal Fixation System | ATEC
March 15, 2021, Alphatec Holdings, Inc. announced the launch of the InVictus® Occipital-Cervical-Thoracic (OCT) Spinal Fixation System, which extends the sophistication of the InVictus Posterior Fixation Platform to the entire spine. The InVictus System is engineered to provide adaptable, predictable surgical treatment of a range of pathologies through open, MIS, or hybrid approaches.
The first new product to launch within ATEC’s posterior cervical portfolio, InVictus OCT minimizes potential tulip splay and cross-threading, utilizing helical flange technology. The system uniquely gives surgeons the option of utilizing single- or dual-rod tulips to create multi-rod constructs that cross the occipito-cervical junction with increased biomechanical stability. To address the challenges commonly associated with crossing the cervico-thoracic junction, the system features multiple favored-angle screws, a variety of rod-to-rod connectors and seamlessly integrating transition rods. To enhance predictability and optionality, the system is fully compatible with the SafeOpTM Neural InformatiX System, Arsenal® Spinal Fixation System, and the InVictus Spinal Fixation System.
Key Features of the InVictus OCT Spinal Fixation System
- Integrates with SafeOp Neural InformatiX System to predictability provide surgeons with real-time, actionable information to detect and monitor the health of nerves at risk during posterior fixation
- Adapts intraoperatively to surgical requirements with robust instruments and customizable implants designed to accept multiple rod diameters and materials, which enables the treatment of more advanced pathologies
- Offers multiple favored-angle screws, a variety of rod-to-rod connectors, and transition rods that work in combination to facilitate crossing the cervico-thoracic junction
- Integrates with InVictus Spinal Fixation System to achieve additional levels of fixation
8.-Stronghold™ 3D Titanium | Spine Wave
March 16, 2021 Spine Wave announced the commercial launch of the Stronghold™ 3D Titanium Interbody Device featuring TiCell™ 3D advanced surface technology. This exciting new titanium lumbar interbody fusion implant is manufactured using a direct metal laser sintering manufacturing process (3D printing) to produce a unique surface intended to optimize the implant’s interface and integration with bone. Stronghold™ 3D Titanium Interbody Device is Spine Wave’s first interbody device featuring TiCell™ 3D advanced surface technology and this new system will substantially broaden the company’s lumbar interbody fusion portfolio and complement Spine Wave’s other innovative spine fusion products.
The Stronghold™ 3D Titanium Interbody Device is offered in both a straight and a pivoting crescent design to comprehensively address the posterior lumbar interbody fusion procedure market. The TiCell™ 3D advanced surface technology featured on all Stronghold™ 3D Titanium Interbody Device implants is characterized by a dual layer design intended to incorporate optimally sized interconnected pores. This interconnected porous structure is constructed in a lattice intended to mimic the organic structure of cancellous bone and provide a rough surface to strengthen the interface between implant and bone. Stronghold™ 3D Titanium Interbody Device implants also feature an open architecture to contain large amounts of autogenous bone graft and allow for implant visualization and fusion assessment on post-operative imaging.
9.-Lumbar Plating Solution | 4WEB Medical
May 5, 2021, 4WEB Medical, announced the initial launch of the newest addition to the company’s portfolio, the Lumbar Spine Plating Solution (LSTS-PS). The first procedures were performed by James Lynch, MD, Neurosurgeon and CEO of Spine Nevada at Renown Medical Center in Reno, NV.
The Lumbar Plating Solution is compatible with the Lateral Spine Truss System™ that incorporates 4WEB Medical’s proprietary Truss Implant Technology™. Under normal loading conditions the struts in the truss implant transfer strain to adjacent cellular material which stimulates a mechanobiologic response.
- Wide variety of modular plating configurations to address multiple lumbar spine pathologies and approaches.
- Integrated and non-integrated plate offering with a one, two and four screw option.
- Plate design features a single-step locking mechanism to prevent screw backout.
10.-ProRay interbody device | Prodorth
May 13th 2001, Prodorth, a medical technology company focused on surgical solutions announced the launch the ProRay interbody device. This Cervical Stand-alone Peek Cage System is one of their most preferred products.
ProRay has the following advanced features:
- Self-Tapping and Self-Locking Screws
- Optimized Screw Angulation
- No need for Anterior Profile
- Cervical Peek Cage with Titanium Screws
- Large Graft Space
- Toothed inferior and Superior Surfaces
- Practical and User Friendly
- Marker for X-Ray

11.-Alicudi-J; Capri-Z; Stromboli-J; Giannutri | Tsunami Medical
May 27th 2021, Tsunami Medical announced the launch of many products of their Second Generation of Spinal Fusion solutions. Its main benefits are the following:
- Excellent Stability:Additive manufacturing technology in combination with a unique geometrical implant design facilitates efficient and reliable primary and secondary fixation.
- Wide Variety of Footprints, Angles & Heights :The unique “net” structure, with its pore size and surface roughness, together with the semi-open internal design allow a large volume of new bone colonisation.
- Additional Built-in Fixation :The additional feature of built-in pins contributes to further enhance primary fixation, as already guaranteed by the excellent stability provided by the “net” structure
Alicudi-J: ALIF 3D Printed Titanium Cage with Built-In Fixation
Capri-Z: ACIF 3D Printed Titanium Cage with Built-In Fixation
Stromboli-J:DLIF 3D Printed Titanium Cage with Built-In Fixation
Giannutri: DLIF 3D Printed Titanium Cage with Expansion Feature
12.-Ortigia-J; Ustica; Procida | Tsunami Medical
June 4th 2021, Tsunami Medical, announced the launch of additional 3D printed cage solutions.Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second Generation of Spinal Fusion solutions.
Ortigia-J: OLIF 3D Printed Titanium Cage with Built-In Fixation
Ustica: TLIF 3D Printed Titanium Cage with Built-In Fixation
Procida:PLIF 3D Printed Titanium Cage with Expansion Feature

13.-RESPONSE™ | OrthoPediatrics
May 28, 2021, OrthoPediatrics Corp. announced the launch of its RESPONSE™ Neuromuscular Scoliosis System. This will be the 36th surgical system the Company has launched.
The system received FDA 510(k) clearance in 2020, and the Company has been preparing for a full-scale domestic launch this summer. RESPONSE Neuromuscular (“RESPONSE NM”) represents the latest addition to the RESPONSE Scoliosis platform, which is designed to treat pediatric patients. This new system is dedicated for the treatment of neuromuscular scoliosis and was developed in conjunction with pediatric orthopedic surgeons to address the distinct challenges in treating a unique patient population. Building on the base of the Company’s RESPONSE Spine System, RESPONSE NM features a complete set of implants and instruments with innovative attributes that simplify insertion and specific options to address extreme hyperlordosis and sacral/pelvic fixation.
The RESPONSE NM system also works in conjunction with other products in the OrthoPediatrics Scoliosis franchise, including the BandLoc Sublaminar banding system, and FIREFLY 3D printed pedicle screw guides. OrthoPediatrics is the exclusive distributor for Mighty Oak Medical’s FIREFLY technology in children’s hospitals across the United States.
14.-CORBEL™ Lateral ALIF Interbody Spacer | Globus Medical
Jun 4, 2021, Globus Medical’s launched an innovative solution for lateral position ALIF surgery! This system features in-line integrated anchor technology to reduce the number of procedural steps compared to screws, offset instruments to simplify midline implant placement, and SintrOS™ surface technology designed to encourage cellular activity at the bone interface. CORBEL™ requires supplemental fixation.
Features and Benefits
- SintrOS™ Surface Technology: In an ovine interbody model, implants with SintrOS™ surface technology demonstrated significantly more bone ongrowth than smooth titanium at 6 weeks postoperatively and was equivalent at 12 weeks.1
1. Data on file at Globus Medical - Protected Surgical Corridor:The MIS anchors are pre-loaded and passed through the holder to help protect patient anatomy.
- Offset Integrated Fixation:Fixation is offset 15° from midline to simplify anchor and screw insertion during the Lateral ALIF approach.
15.-Idys®ALIF 3DTi; Idys®ALIF ZP 3DTi; Idys®LLIF 3DTi | Clariance Spine
June 15th, 2021, Clariance, announceD the completion of their anterior and lateral range and its global launch.On April 12th, 2021, the company received 510k clearance for their product Idys®-ALIF 3DTi, completing their anterior and lateral range package in the United-States.
Following this news, on May 25th, 2021 they received five new CE0459 certifications from the GMED in Europe. These approvals will enable Clariance to commercialize a full package of solutions for the anterior
and lateral range, adding to their equally comprehensive cervical range.
Clarisse Demarcq, Marketing Director, explains : “For surgeons who want a purely anterior approach with maximum stability, we have developed the Idys®-ALIF 3DTi which includes a secure cage, a plate, and 4 screws.”
Clariance also offers a zero-profile approach with the Idys®-ALIF ZP 3DTi, with four fixed-angle screws, designed to ensure an optimal grip.
For a lateral approach, Clariance has designed the Idys®-LLIF 3DTi, an implant that also uses an intelligently designed plate that allows for adapted screw angulation by the surgeon. Additionally, in order to provide the best possible comfort for the practitioner in this approach, Clariance has designed its own easy to use CLARVIEW retractor.
The whole range is printed in 3D Titanium, and each of these three products uses the same self-tapping spinal screws. “Our range has been designed in collaboration with surgeons from various backgrounds in
order to meet the needs of all practitioners and techniques in use,” concludes Mrs. Demarcq.
16.–3D-Printed FORZA Titanium PLIF Spacer System with Nanovate Technology | Orthofix
June 29, 2021, Orthofix Medical announced the U.S. launch and first patient implant of the FORZA™ Ti PLIF Spacer System. Developed to enhance Posterior Lumbar Interbody Fusion (PLIF) procedures, the 3D-printed FORZA Ti Spacer with Nanovate™ Technology is a titanium lumbar interbody device featuring an optimized design, porosity and surface that allows bone to grow into and through the spacer.
Features of the FORZA Ti PLIF Spacer System include:
- Large open graft window for packing bone-grafting material
- Bulleted nose to assist with distraction
- 3D-printed porous titanium with macro, micro, and nanoscale surface features
- Nanoscale surface that has been shown to increase proliferation and alkaline phosphatase activity (an early osteogenic differentiation marker) in human stem cells in vitro*
- Functional gradient porous structure with 80-percent porosity at the midline of the implant which allows for increased fluoroscopic visualization
- Endplates with 400 micron pores and 50-percent porosity designed to help facilitate bone ingrowth**
- Endplates consisting of interconnected gyroid structures analogous in form to trabecular bone which provide an open porous environment
The FORZA Ti PLIF Spacer System with Nanovate Technology is available in the U.S. through a targeted commercial release.
17.-Modulus® ALIF | Nuvasive
July 22, 2021, NuVasive,announced today the commercial launch of Modulus® ALIF, a 3D-printed porous titanium implant for anterior lumbar interbody fusion (ALIF), in targeted global regions.
Modulus ALIF is the latest addition to the NuVasive Advanced Materials Science® (AMS) portfolio and provides implants and instrumentation designed for both supine ALIF and XALIF™ procedures. In addition, Modulus ALIF comes in a variety of sizes and lordotic options to accommodate varying patient anatomy. Highlights of the Modulus ALIF technology include:
- Proprietary Modulus titanium design: Modulus is designed for enhanced osseointegration,1 biomechanical,1 and imaging properties. The porous surface architecture is engineered to participate in fusion and promote new bone on-growth and in-growth.2 The proprietary design also allows for enhanced visualization compared to solid titanium implants.
- Zero-step locking mechanism: This feature provides definitive tactile and visual confirmation, enabling confidence in a surgeon’s screw placement in the implant.
- Low-profile, versatile instrumentation: The device’s instrumentation can be utilized in both supine ALIF and XALIF, supporting a surgeon’s preferred surgical approach. The instrumentation’s low-profile and versatile features are designed to increase operating room workflow efficiencies.
18.-WaveForm A interbody | Seaspine
Aug. 12, 2021 SeaSpine announced the limited commercial launch of its 3D-printed WaveForm A (ALIF) Interbody System.
WaveForm A is designed for the ALIF (Anterior Lumbar Interbody Fusion) procedure, and seamlessly integrates with the entire Meridian anterior interbody portfolio. WaveForm A delivers a fully porous interbody solution with a graft aperture designed to accommodate a large amount of SeaSpine’s best-in-class allograft demineralized bone matrix offerings OsteoStrand® and OsteoStrand® Plus.
The WaveForm A interbody offers the next level of 3D-printed architectural innovation, balancing key geometric and manufacturing advancements without compromising clinical requirements. WaveForm A utilizes innovative WaveForm technology to deliver a highly porous and robust interbody solution. This design is intended to balance subsidence resistance, implant stiffness, and orthobiologics packability, while maintaining radiographic visualization during intraoperative and postoperative imaging.

19.-FlareHawk7 | Accelus
Aug. 17, 2021, Accelus announced the launch of FlareHawk7, a multidirectionally expandable lumbar fusion device that is inserted at an ultra-low profile of 7mm to help minimize neural retraction before expanding in both the cranial-caudal and medial-lateral planes to provide sagittal and coronal correction, foraminal height restoration, and the stability to promote fusion.
The FlareHawk7 platform also features a suite of instruments designed to facilitate surgeons’ technique preferences. For endoscopic TLIF procedures, surgeons can leverage access with instruments to allow direct visualization of disc preparation and implant delivery. For MIS procedures, surgeons are provided access to the disc space through Kambin’s triangle while also preserving the patient’s normal anatomy. And in a “mini-open” procedure, the need for neural retraction can be minimized thanks to the smaller insertion footprint that is similar in profile to a No. 2 pencil.
FlareHawk7 permits concurrent expansion in height and width to restore disc height without sacrificing stability. It enters the disc space with a compact 7mm x 7mm profile and then expands up to 11mm x 12mm—a 57% increase in width and 72% increase in height—with 0° and 6° lordosis options. This provides a larger surface contact area to distribute load with the goal of reducing subsidence in weak, osteoporotic bone. The implant’s open architecture design also allows significant graft delivery through the implant and into the surrounding disc space.
20.-Luna XD and Orbit | Spinal Elements
September 15, 2021, Spinal Elements announced the full commercial launch of the Luna XD multi-expandable lumbar interbody fusion device and Orbit articulating discectomy systems. Luna XD and Orbit have been integrated into Spinal Elements as the newest technologies in its MIS Ultra™ platform of products and procedural solutions.
The Orbit system consists of articulating and rotating discectomy instrumentation that uses a minimal, posterior incision and/or MIS tubular approach to achieve efficient disruption and removal of disc tissue while preparing the endplates for fusion. The novel articulating rotary design allows the instrumentation’s working end to evacuate the full area of the disc necessary for implantation of the Luna XD circular device.
The Luna XD system allows an ALIF-sized, circular implant to be surgically delivered to the intervertebral space through a posterior, minimally invasive access point. Once inserted, the Luna XD implant first expands horizontally to cover the disc footprint, creating a stable foundation. The implant then expands vertically up to 16mm to restore disc height. Furthermore, the device includes up to 12° of lordosis to aid in the restoration of sagittal alignment.
21.-SPIRA-P Posterior Lumbar Spacer; SPIRA-T Oblique Posterior Lumbar Spacer | Camber Spine
September 22, 2021 Camber Spine initiated the full national launch of its SPIRA-P Posterior Lumbar Spacer and SPIRA-T Oblique Posterior Lumbar Spacer devices.
News of the national launch comes on the heels of Camber’s announcement last month that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for both products, which are indicated for use in skeletally mature patients with Degenerative Disc Disease (DDD) at one or two contiguous levels from L2-S1.
Part of the SPIRA® product platform, the SPIRA-P Posterior Lumbar Spacer can be utilized to accommodate PLIF or TLIF procedures and features a patented open architecture design for optimal endplate load distribution. Plus, its uniquely designed surface allows for cell adhesion and bone cell proliferation while its interconnected porosity design mimics bone.
The SPIRA-T Oblique Posterior Lumbar Spacer features the same qualities, but is designed specifically to accommodate traditional or “insert and rotate” TLIF procedures. Uniquely, its shape is angled for a 25° oblique insertion technique to optimize lordosis.
As with all products within Camber’s SPIRA technology platform, SPIRA-P and SPIRA-T include strategically placed and optimal sized openings for graft packing. The SPIRA products are designed to decrease the risk of subsidence with optimized endplate distribution and provide good visibility for fusion. The devices’ bone-like surface design promotes “mechanical fusion” bone ingrowth for short term stability and optimized biomimetic scaffolding designed to encourage osteogenesis.
22.-Advantage-C™ PEEK Cervical Interbody Fusion Device | Intelivation Technologies
Sept. 24, 2021, Intelivation Technologies announced that they are officially launching the Advantage-C™ PEEK Interbody Fusion Device during the 36th Annual NASS Meeting in Boston September 29-October 2, 2021.
The Advantage-C™ device is designed to be used in skeletally mature patients in levels C2-T1 in conjunction with fixation for ACDF (anterior cervical discectomy and fusion) procedures. Advantage-C™ was developed to optimize fusion, while maintaining elasticity that is similar to bone and radiolucency of the cage body. President Amit Sinha stated, “We are looking forward to a successful launch of Advantage-C™ and the myriad of efficiencies this device will bring to both surgeons and hospital systems. Advantage-C is the basis for our rapidly growing interbody platform that will see continued innovation and product releases over the next year.” CEO Rob Anderson added, “Our growing line of interbody devices further drives home our commitment to bring cost-effective spinal solutions that consistently provide superior clinical results to the market. On behalf of the executive team, I am very impressed how quickly our R&D team worked to bring this device to market.”
23.-Salvo® 5.5/6.0mm Spine System | Spine Wave
Sept. 27, 2021, Spine Wave announced the successful completion of its limited market release and subsequent launch of the Salvo® 5.5/6.0mm Spine System featuring Spine Wave’s advanced modular screw design. Spine Wave launched the Salvo® 4.75mm Spine System during late 2020 to address cortical bone trajectory and low-profile transpedicular procedures. Now, the Salvo® 5.5/6.0mm Spine System is available for procedures performed with 5.5mm and 6.0mm rods, which are the most often used for thoracolumbar spine fixation procedures. The Salvo® Spine System, now available with 4.75mm, 5.5mm and 6.0mm titanium and cobalt chromium rods, will substantially strengthen Spine Wave’s position in the thoracolumbar fixation market and complement the company’s broad portfolio of innovative spine fusion technologies.
The Salvo® 5.5/6.0mm Spine System addresses most thoracolumbar fixation procedures with an advanced modular screw design whereby the separate “tulip” and “shank” components of the screw can be assembled in-situ after surgical decompression or on the back table prior to implantation. This versatility can enhance visibility within the surgical field even with less invasive procedures. Surgeons appreciate the distinct audible and visual keys that make confirmation of proper screw assembly easy and reliable. The novel modular tensioned head prevents the tulip from “flopping”, which facilitates rod approximation into the screws. Taken together, these features can make thoracolumbar fixation procedures easier, faster, and safer. Hospitals also appreciate Salvo® Spine System’s modular design because it can provide a full complement of implant options with fewer cases and trays to process and keep on site, which may reduce their operating costs.
24.- Golden Isles Pedicle Screw System™ | Intelivation Technologies
Sept. 28, 2021, Intelivation Technologies, announced the official launch of the Golden Isles Pedicle Screw System™ during the 36th Annual NASS Meeting in Boston September 29-Ocotber 2, 2021.
The Golden Isles Pedicle Screw System™ is designed to provide immobilization and stabilization of spinal segments in skeletally mature patients in treating the lumbar and sacral spine. The system is intended for posterior, non-cervical pedicle fixation in skeletally mature patients for treating degenerative disc disease, spondylolisthesis, trauma, deformities as well as pseudoarthrosis and failed previous fusions.
25.- Duo Ti Expandable Interbody Fusion Procedure | Spineology
Sept. 28, 2021 Spineology announced the launch of the Duo Ti Expandable Interbody Fusion Procedure, which combines Spineology’s proprietary mesh technology with porous titanium to deliver a large, anatomy conforming implant via anatomy-conserving lateral decubitus and prone approaches. The porous titanium blocks are made from OsteoSync™ Ti (SITES Medical) and feature a highly porous macro structure with micro and nano-texturing, and a low modulus of elasticity to facilitate superior bone ingrowth capabilities.
The Duo Ti procedure is performed through a small portal tube. Following insertion of the implant, the mesh component is filled and expanded with bone in situ which enables surgeons to reduce retraction requirements, avoid and protect neural structures, and indirectly decompress. The result for patients and care providers is enhanced recovery, proven outcomes backed by Spineology’s 200-patient Duo Clinical Study (RaDical), and exceptional efficiency.1
- Enhanced Recovery: According to RaDical, subjects did not experience clinically meaningful new onset or increased thigh symptoms (i.e., pain, hip flexion/extension weakness) following the Duo procedure. Less trauma to the psoas muscle decreases or eliminates the risk of indirect nerve root injury and may accelerate patient recovery.
- Proven Outcomes: At three months, individual patients in the RaDical study showed a 71 percent improvement in their lower extremity pain and 57 percent improvement in their low back pain. At 12 months, 98 percent of patients had fused.
- Exceptional Efficiency: Duo can be performed as a single-position prone procedure which eliminates the need to flip a patient intraoperatively and saves surgeons operative time, which can lead to overall reduced procedure costs.
26.- Angled Endplate implants for the prodisc® L | Centinel Spine
Sept. 28, 2021, Centinel Spine® announced the launch of Angled Endplate implants for the prodisc® L Lumbar Total Disc Replacement System. These new endplates have been designed to shift the lordotic angle of the implant to the inferior endplate, expanding the options available to surgeons to better address the varied lumbar anatomy and pathology of patients. The Angled Endplates will be featured during the upcoming North American Spine Society (NASS) 36th Annual Meeting (September 29-October 2, 2021, Boston, MA).
Centinel Spine continues to innovate in the total disc replacement market, and the release of these Angled Endplates in the U.S. represents one of several major recent achievements, including FDA approval of two-level indications for the prodisc L system. The launch of the Angled Endplates includes six angled options, including inferior endplates with lordotic angles of 3° and 8° and a superior endplate with a lordotic angle of 3°.
The new prodisc L Angled Endplate implants will be featured at booth (#1612) during NASS 2021. The prodisc L Total Disc Replacement System will also be featured during a podium presentation by Thierry Marnay, MD, reporting on new study results that strongly support the long-term safety and effectiveness of prodisc L for the lumbar spine. Results from this study will be presented during the Best Paper session on Thursday, September 30, 2021.
27.- InVictus OsseoScrew Expandable Spinal Fixation System | ATEC
Sept. 29, 2021, Alphatec Holdings, Inc. announced the launch of the InVictus OsseoScrew Expandable Spinal Fixation System.InVictus OsseoScrew has been designed to be an alternative to the conventional use of cemented fenestrated screws for patients with compromised bone. The system is intended to restore spinal column integrity, even in the absence of fusion, in patients with advanced stage tumors involving the thoracic and lumbar spine in whom life expectancy is of insufficient duration to permit achievement of fusion. An expansion of the InVictus Posterior Fixation Platform, InVictus OsseoScrew integrates seamlessly with InVictus MIS and Open platforms as well as SafeOp™ Automated EMG.
28.- RODIN™ | CTL Amedica
Sept. 29, 2021, CTL Amedica Corporation unveiled its RODIN™ Transforaminal Expandable Lumbar Interbody Fusion System during the North American Spine Society (NASS) annual meeting and exhibition. FDA-cleared and in beta launch at CTL Amedica, RODIN™ is a horizontally expandable, minimally invasive interbody implant made of titanium. It has superior biomechanics, surface area and graft volume capacity, all with a simplified delivery system. At a 6mm cross-sectional width, non-deployed, it offers one of the smallest collapsed footprints of any expandable on the market. With its horizontally expanding capabilities, convex surfaces and lordotic offerings, the RODIN™ accomplishes a stable and impressive ALIF footprint through an MIS posterior approach.
CTL Amedica will launch RODIN™ with a long term (> 10-year follow-up) clinical series with a 98.5% clinical and radiographic fusion rate and an animal study showing 100% fusion throughout the entire series, with post study CT scan and histologic confirmation. Dr. Fabian has implanted the product in multiple surgeries with excellent results.
29.- NEXXT MATRIXX® Cervical Stand-Alone System | Nexxt Spine
Sept 29, 2021, Nexxt Spine, announced the commercial launch of the NEXXT MATRIXX® Cervical Stand-Alone System. Featuring technology unique to the NEXXT MATRIXX® line of products, this thoughtfully engineered 3D laser printed titanium implant includes varying pore sizes from 300μm, 500μm and 700μm. The NEXXT MATRIXX® Cervical Stand-Alone System provides the strength and biocompatibility of titanium and a modulus of elasticity comparable to PEEK.
The NEXXT MATRIXX® Cervical Stand-Alone System is a minimal access technology that promotes less trauma for the patient and, by eliminating the need for a supplemental fixation plate, saves valuable time for the surgical team. Built on the NEXXT MATRIXX® five pillars for successful fusion, the additively manufactured device provides the essential framework for successful anterior cervical discectomy and fusion (ACDF) procedures. The NEXXT MATRIXX® Cervical Stand-Alone System includes multiple footprints, heights, and lordosis options – each with integrated fixation screws – and is designed to maximize safety by minimizing retraction and exposure.
30.- Catalyft™ PL and PL40 | Medtronic
Sept. 30, 2021, Medtronic announced the latest additions to its minimally invasive spine surgery ecosystem: Catalyft™ Expandable Interbody System. Catalyft™ PL and PL40 feature a unique design for anterior rim engagement, a beveled tip for ease of insertion, seamless integration with StealthStation™ Navigation, simplified bone graft delivery, and active expansion at the precise angle and lift that surgeons need for minimally invasive, patient-specific solutions to meet sagittal alignment goals.
31.- NorthStar OCT | Seaspine
Oct. 11, 2021, SeaSpine announced the full commercial launch of its NorthStar OCT Posterior Cervical Fixation System. The launch of NorthStar OCT represents the Company’s first full commercial introduction of a new posterior cervical system since 2007 and provides a differentiated and more robust product offering to address a market segment that SeaSpine estimates to exceed $250 million in the United States alone.
NorthStar OCT was designed to expand versatility, offering both clinical and economical efficiencies, and brings to market an innovative and elegant option to treat a wide range of patient pathologies, including complex cervicothoracic deformity. The system’s novel instrumentation and anatomically designed implants provide a safe and effective solution designed to improve surgical flow when navigating through complex procedures. Some of the more differentiated features include shingled occipital plates with variable screws and a novel drill and screw guide that provides improved access during occipital screw placement, high angulation screws that ease rod placement, and pedicle-specific screws with dual-lead threads.
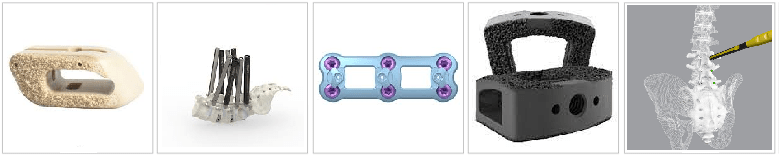
32.- Cohere TLIF-O | Nuvasive
Oct. 14, 2021, NuVasive announced the launch of the Cohere® TLIF-O implant and upcoming launch of the Cohere TLIF-A implant planned for later in the fourth quarter. With these additions to its Advanced Materials Science™ (AMS) portfolio, NuVasive is the only company to offer both porous PEEK and porous titanium implants for posterior spine surgery.1
Cohere TLIF-O is a porous PEEK implant for the transforaminal lumbar interbody fusion (TLIF) procedure with a variety of implant sizes to accommodate various patient anatomies and procedurally designed instrumentation. Its design adheres to NuVasive’s AMS principles of surface, structure, and imaging and includes the following features:
- Surface: The implant’s proprietary porous surface technology generates stronger integration through bony in-growth and on-growth2 in comparison to smooth PEEK,3,4 which aids fusion and overall clinical outcomes;
- Structure: Cohere TLIF-O is designed with lordosis in the oblique plane which helps maintain coronal alignment while restoring sagittal balance; and
- Imaging: The radiolucent polymer composition of porous PEEK enables clear radiographic visualization of the fusion site with a variety of different imaging modalities.
33.- Mariner MIS Wayfinder | Seaspine
Nov. 01, 2021, SeaSpine announced the limited commercial launch and completion of initial surgeries of the Mariner® MIS Wayfinder System.
Mariner MIS Wayfinder is a novel, one step, k-wireless screw delivery system for pedicle screw fixation. Mariner MIS Wayfinder eliminates reliance on traditional guidewires for percutaneous screw placement by providing a fully-integrated, surgeon-led solution that enables real-time feedback. This most recent launch reflects SeaSpine’s continued expansion of its Mariner platform and builds on the momentum from the limited commercial launch of the Mariner Adult Deformity System.
The Mariner MIS Wayfinder System embodies the design principles integral to the Mariner core philosophy, providing clinicians an innovative and intuitive approach to pedicle screw fixation, whether using traditional fluoroscopy or SeaSpine’s 7D FLASH Navigation System. “In the initial cases, the Mariner MIS Wayfinder System has already demonstrated significant efficiencies, particularly when paired with 7D technology,” said Shaeffer Bannigan, Senior Director of Product Development.
34.- Defender® Anterior Cervical Plate, Stronghold® C 3D | Spine Wave
Dec. 02, 2021, Spine Wave announced the immediate launch of both the Defender® Anterior Cervical Plate, and the Stronghold® C 3D Titanium Interbody Device featuring TiCell® 3D advanced surface technology. The Defender® Anterior Cervical Plate is a titanium plate and screw system that provides fixation for anterior cervical fusion procedures. The Stronghold® C 3D Titanium Interbody Device is a cervical interbody fusion implant manufactured using 3D printing methods to produce a unique surface designed to optimize the implant’s interface with bone. These exciting new products substantially strengthen Spine Wave’s position in the important anterior cervical fusion market segment.
The Defender® Anterior Cervical Plate is a “workhorse” titanium anterior cervical fixation system for anterior cervical fusion. It provides secure fixation by offering fixed and variable angle screws in a dual- lead thread design in multiple diameters with a wide range of lengths, and in both self-drilling and self-tapping designs. Simple locking is provided with a dual-cam locking mechanism that secures both screws at each level in one step. The Defender® Anterior Cervical Plate is also designed with large graft windows to provide direct visualization during the procedure and to facilitate radiographic visualization after the procedure.
The Stronghold® 3D Titanium Interbody Device is offered in a wide range of sizes to comprehensively address the requirements of cervical interbody fusion procedures. The TiCell® 3D advanced surface technology featured on all Stronghold® 3D Titanium Interbody Device implants is characterized by a dual layer of optimally sized interconnected pores. This interconnected porous structure is constructed in a lattice intended to mimic the organic structure of cancellous bone and provide a rough surface to strengthen the interface between implant and bone. All Stronghold® 3D Titanium Interbody Device implants, which are also offered in TLIF and PLIF designs in addition to Cervical, also feature an open architecture to contain large amounts of bone graft and facilitate fusion.
35.- CentraFix | CoreLink
Dec. 2, 2021, CoreLink announced the commercial launch and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the CoreLink CentraFix™ Midline Fixation System.
CentraFix is a posterior thoracolumbar pedicle screw system designed for less invasive spinal fixation often used with a medial-to-lateral approach, known as cortical bone trajectory (CBT). This technique maximizes contact of the pedicle screw with cortical bone and is intended to reduce incision size, limit muscular and vascular injury, and improve initial fixation. CentraFix features modular cobalt chrome tulip heads and titanium alloy screw shanks in various lengths and diameters, designed specifically to allow screw placement in denser cortical bone.
The CentraFix low-profile, modular tulip heads have been designed to minimize tissue disruption and simplify distraction without compromising strength. Self-drilling, self-tapping cortical screw threading provides easy screw starting in the intended trajectory and allow for 360° motion with a 60° cone of angulation. The system includes 4.75mm and 5.5mm rod options, set screws designed to minimize tulip splay, and a robust offering of ergonomic and intuitive instrumentation to facilitate fast and efficient surgery.
NOTE:
This information is for educational purposes only! It is not intended to infringe copyright. All documents are of the exclusive property of the manufacturing companies.We hope you find this information valuable and useful. We will try to update it often! We invite you to send us in pdf format the brochures and surgical techniques that we lack! Thanks in advance! Please send them to: spinemarketgroup@gmail.com
