NEST Interbody System is a hollow, rectangular implant that consists of a unique configuration of both solid and porous structures, which are built using Paonan’s 3D printing Titanium technology.
The system was designed for bone in‑growth and biological fixation. Inspired by the microstructure of cancellous bone, and enabled by Paonan’s 3D printing manufacturing process, this technology is deliberately designed for fusion.
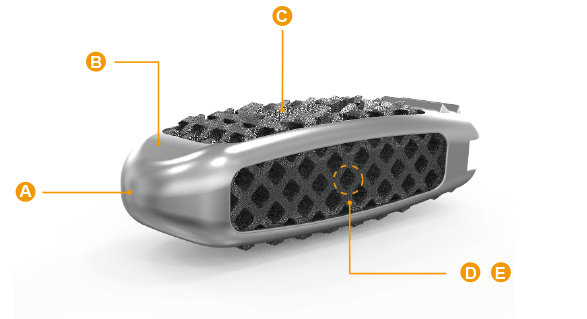
- A. Smooth Wedge-shape Design:Easy Insertion
- B. Titanium Dioxide Layer and Rough Surface: Promote cellular adhesion and proliferation
- C. Serrated Surface Design: Enhance fixation stability and avoid expulsion/migration
- D. Diamond Lattice / Solid Framework Composite Architecture:Promote cellular adhesion and proliferation
- E. Interconnected Porous Structure
- Not only on-growth; in-growth as well
- Created to allow radiographic visualization
*Pore size range: 700~900µm / Porosity: ≈70~80%
Optimal imaging characteristics:Nest Interbody System has excellent imaging properties on X-Ray and CT.
About Biomech Spine
The world 1st case of spinal implant was carried out in 1984, and the following year BIOMECH Paonan was established. The company’s founder, Benny Yeh has more than 30 years’ experience in designing and marketing spinal devices, and participated in more then 200 patent application. BIOMECH Paonan is always in the trend with the spinal device technology. Our spinal products include FUSION and NON-FUSION treatment. We fulfill clinical unmet needs by designing delicate devices which are easy to use.
BIOMECH Paonan’s R&D team collaborate with international medical advisors to provide the best quality products and services. Furthermore, we also establish several service branches in Asia region.
BIOMECH Paonan’s products include:
● Cervical, Thoracic, Lumbar pedicle screws
● ALIF, PLIF, TLIF cages
● Facet fusion device
● Interspinous decompression device
● Interspinous fixation device
● Cervical artificial spacer
● Dynamic fixation
● External fixation system
BIOMECH Paonan have different spinal products to treat different indication of spine. The company is still expanding and growing, we welcome all who are dedicated to this medical field to join us.
PAONAN has been 25 years experience in design orthopedic products. Our R&D team not only have surgery experience but also biomechanical and biomedical engineers. We dedicated to develop external fixation and spine products, especially in totally spine solutions. www.biomech-spine.com
