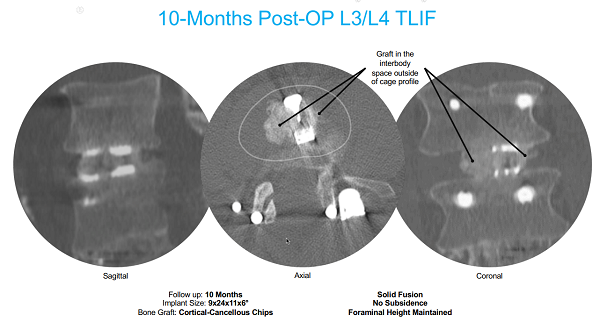
July 29, 2024 — Incline Village, Nev., — “This patient scan 10 months post-surgery utilizing the KG®2 Surge® for a TLIF procedure shows obvious fusion, and demonstrates the value of a biologic foundation of graft between vertebral end plates, maintaining disc height, preventing implant subsidence and preservation of foraminal height”, said Jeff Kleiner, MD, founder and CEO of Kleiner Device Labs. “The importance of that biologic foundation was highlighted in the recent release of a study by the University of Toledo into the higher failure rate of spinal fusions in diabetic patients.”
The full study: doi.org/10.1093/jbmrpl/ziae053.
Added Nathan Wanderman, MD, of Twin Cities Orthopedics in Egan, Minnesota, “I have been using KG 2 for a year and have yet to encounter a pseudoarthrosis. I have been surprised at how much more graft I can get into disc spaces with the device, and there is an appreciable difference in graft volume compared to products I have used in other settings.”
The new KG2 Surge flow-thru interbody system was developed with the objectives of maximizing bone graft delivery to the prepared intervertebral disc space, and streamlining implant placement, positioning, and integration in the graft matrix.
Media Contact
Brad Samson
brad.samson@kleinerlabs.com
M: 714.955.3951
MKT-00010-78 Rev 1
