Plano, TX, September 06, 2024 –(PR.com)– The Eminent Spine Scoliosis Deformity Pedicle System consists of rods, polyaxial screws with set caps, and cross connectors with locking screws.
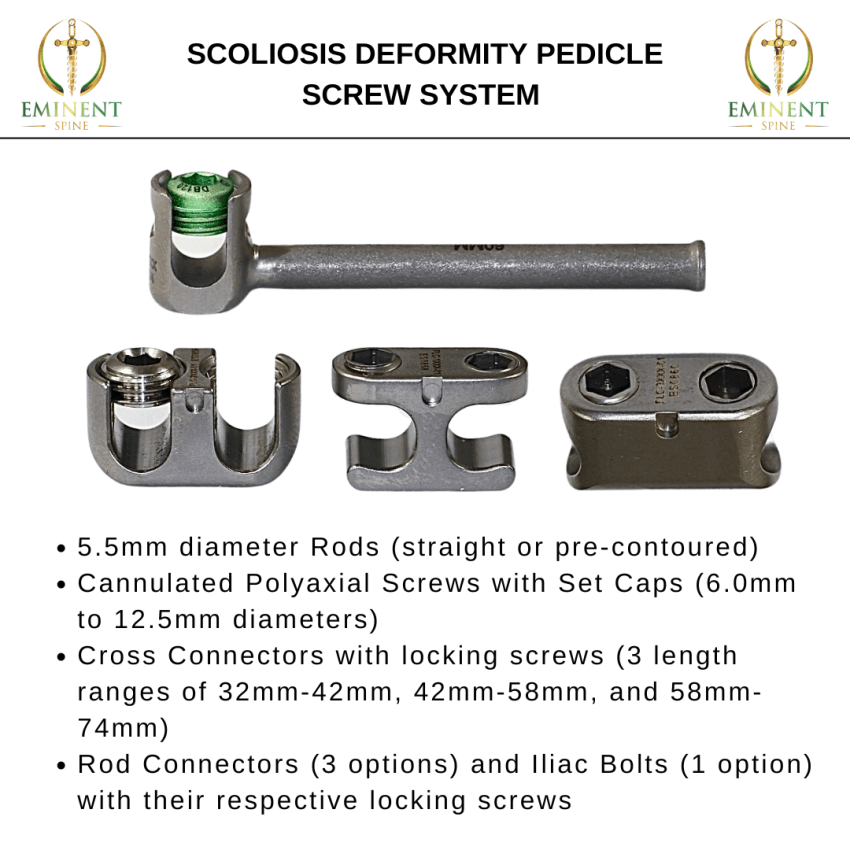
Additionally, the system consists of rod connectors and iliac bolts with their respective locking screws. Rods are 5.5mm in diameter and are available either straight or pre-contoured. Straight and pre-contoured rods are each offered in lengths ranging from 40mm to 600mm in various increments. Cannulated polyaxial screws are available in 6.0mm to 12.5mm diameters and in lengths ranging from 40mm to 110mm in 5mm increments. Set caps are used to fasten the rod and screw. Cross connectors are available in 3 length ranges: 32mm-42mm, 42mm-58mm, and 58mm-74mm. Cross connectors lock screws are used to fasten the cross connector together and fasten across the rods. Rod connectors are offered in 3 types and the iliac bolts are offered in 1 type. All of the components are available in a variety of sizes to match more closely to the patient’s anatomy. All components are made from titanium alloy per ASTM F136.
Contact
Eminent Spine
Dagen Hybner
972-499-3593
www.eminentspine.com
