Today, everybody say that AI and robotics will change everything. However, this type of prediction is not new. The world and our industry has heard similar promises before. More than ten years ago, when 3D printing started, the narrative was very similar: manufacturing would become fully additive, implants would be patient-specific, and hospitals would print their own … [Read more...] about The Spine Industry and Additive Manufacturing: Still Waiting for the Future?
NEWS
How the Brochure Section of SPINEMarketGroup Can Help You in Your Daily Work?
In the fast-moving spine device industry, reliable technical documentation is essential — which is why our Brochures section has become a trusted industry reference. Whether you are a surgeon, sales representative, product manager, engineer, or researcher, this section offers a centralized library of spine product brochures and surgical techniques that can save you time and … [Read more...] about How the Brochure Section of SPINEMarketGroup Can Help You in Your Daily Work?
Gad Medical Ltd. Receives Regulatory Approval to Start Sales of Ruthless Spine’s RJB Interoperative Angle Measurement Instrument in Israel, Marking Significant Expansion Following NASS Momentum
LOS ANGELES--(BUSINESS WIRE)--Ruthless Spine today announced that Gad Medical Ltd. has received regulatory approval to immediately start sales in Israel of Ruthless Spine’s RJB interoperative angle measurement instrument. This development follows their agreement on distribution rights to the RJB, enabling hospitals and surgical centers to access a U.S.-engineered implant … [Read more...] about Gad Medical Ltd. Receives Regulatory Approval to Start Sales of Ruthless Spine’s RJB Interoperative Angle Measurement Instrument in Israel, Marking Significant Expansion Following NASS Momentum
Inside Brazil’s $450M Spinal Implant Market: Key Local Players and Regulations
The spinal implants market in Brazil is a significant niche within the medical device industry, showing steady growth in recent years. According to 2024 reports, the spinal implants and devices market in Brazil generated approximately USD 400-450 million. It is projected to reach USD 850 million by 2033, with a compound annual growth rate (CAGR) of 7.5% between 2025–2033.Within … [Read more...] about Inside Brazil’s $450M Spinal Implant Market: Key Local Players and Regulations
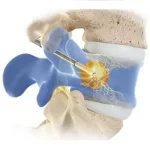
Boston Scientific announces positive coverage policy for the Intracept™ Procedure by Health Care Service Corporation
New policy expands patient access to Intracept Procedure for chronic vertebrogenic back pain, covering 26 million lives What’s New: Boston Scientific today announced that Health Care Service Corporation (HCSC), one of the largest commercial health insurers in the U.S., now covers the Intracept™ Procedure for eligible patients with chronic vertebrogenic low back pain. … [Read more...] about Boston Scientific announces positive coverage policy for the Intracept™ Procedure by Health Care Service Corporation
Providence Medical Technology, Inc. Announces Compatibility of CORUS-GLX™ System with Globus Medical’s Excelsius™ Navigation System for Posterior Lumbar Fusion
PLEASANTON, Calif., Dec. 9, 2025 /PRNewswire/ -- Providence Medical Technology, Inc., an innovator in solutions for spinal surgery, announces that its CORUS™ Navigation Access System-GLX ("CORUS-GLX System") is now compatible with Globus Medical's ExcelsiusGPS™ Robotic Navigation Platform for use during spinal fusion surgery. This new compatibility clearance … [Read more...] about Providence Medical Technology, Inc. Announces Compatibility of CORUS-GLX™ System with Globus Medical’s Excelsius™ Navigation System for Posterior Lumbar Fusion
With the C Disc cervical prosthesis,Cousin Surgery strengthens its range of implants dedicated to preserving mobility
Wervicq, France - December 10. 2025--Cousin Surgery, a major player in implantable medical devices, is expanding its range of spinal surgery products with the acquisition of C Disc, the first reverse cervical prosthesis with a double centre of rotation. The innovative design and exclusive biomechanical performance of this implant enables surgeons to preserve and control … [Read more...] about With the C Disc cervical prosthesis,Cousin Surgery strengthens its range of implants dedicated to preserving mobility
FFX® Receives FDA Clearance for Cervical Facet Arthrodesis, Expanding its U.S. indications
Strasbourg, France – December 8, 2025 – SC Medica today announced that its FFX® Facet FiXation System has received U.S. Food and Drug Administration (FDA) clearance for cervical use, marking a significant expansion of its clinical indications in the United States. Following the recent FDA authorization of FFX® for standalone lumbar facet … [Read more...] about FFX® Receives FDA Clearance for Cervical Facet Arthrodesis, Expanding its U.S. indications
Commentary on “Is Globus Medical (GMED) a Solid Growth Stock? 3 Reasons to Think ‘Yes’”
Today I read an article titled “Is Globus Medical (GMED) a Solid Growth Stock? 3 Reasons to Think ‘Yes’, and I found it interesting enough to comment on. The article explains why Globus Medical may be an attractive option for growth-oriented investors, focusing on earnings growth, strong cash flow, and positive revisions to earnings estimates. While it presents a convincing … [Read more...] about Commentary on “Is Globus Medical (GMED) a Solid Growth Stock? 3 Reasons to Think ‘Yes’”
SpineGuard Obtains the Clearance of Three New PediGuard® Models in China
PARIS and BOULDER (CO), December 4, 2025 – 6:00 pm CET - SpineGuard (FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced that the Chinese authorities (NMPA) have cleared the Curved and XS PediGuard models, as well as a … [Read more...] about SpineGuard Obtains the Clearance of Three New PediGuard® Models in China
MiRus Announces First Cases with EUROPA® Posterior Cervical Fusion System with New Technology Add-on Payment (NTAP)
ATLANTA, Dec. 3, 2025 /PRNewswire/ -- MiRus is excited to announce that patients who benefit from health coverage through Centers for Medicare and Medicaid Services (CMS) have been the beneficiary recipients of the latest technology called EUROPA® Posterior Cervical (PCF) System, based on it's proprietary rhenium alloys, for treatment of the cervical and upper … [Read more...] about MiRus Announces First Cases with EUROPA® Posterior Cervical Fusion System with New Technology Add-on Payment (NTAP)
Carlsmed Announces U.S. Launch of its aprevo® Technology Platform for Cervical Fusion Surgeries at the Cervical Spine Research Society (CSRS) 53rd Annual Meeting
CARLSBAD, Calif., Dec. 03, 2025 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), today announced the U.S. commercial launch of its aprevo® Technology Platform for cervical fusion surgeries. Surgeons involved in the clinical evaluation will also present early clinical data at the Cervical Spine Research Society (CSRS) 53rd Annual Meeting in … [Read more...] about Carlsmed Announces U.S. Launch of its aprevo® Technology Platform for Cervical Fusion Surgeries at the Cervical Spine Research Society (CSRS) 53rd Annual Meeting
PathKeeper Surgical Receives Innovative Technology Contract from Vizient for Spine Navigation System
Contract awarded for products that bring improvement to healthcare industryGROTON, CT, UNITED STATES, December 2, 2025 /EINPresswire.com/ -- PathKeeper Surgical announced its Spine Navigation System has received an Innovative Technology contract from Vizient®, the nation’s largest provider-driven healthcare performance improvement company. The contract was awarded based on … [Read more...] about PathKeeper Surgical Receives Innovative Technology Contract from Vizient for Spine Navigation System
Coverage Expansion Continues for One- and Two-Level Lumbar Total Disc Replacement as One of the Leading Commercial Payers in Arizona Establishes Positive Coverage Criteria for Centinel Spine’s prodisc® L
WEST CHESTER, Pa., Dec. 2, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced a significant coverage advancement for … [Read more...] about Coverage Expansion Continues for One- and Two-Level Lumbar Total Disc Replacement as One of the Leading Commercial Payers in Arizona Establishes Positive Coverage Criteria for Centinel Spine’s prodisc® L
Expanding Innovations Announces Leadership Realignment to Support Record Growth and Upcoming Product Launches
MOUNTAIN VIEW, Calif., Dec. 2, 2025 /PRNewswire/ -- Expanding Innovations™ (EI), an emerging leader in expandable implant technology for spine surgery, today announced a strategic leadership transition designed to support the company's accelerating growth and expanding operational needs. Ron Sacher, Co-Founder and Executive Chairman of Expanding Innovations, … [Read more...] about Expanding Innovations Announces Leadership Realignment to Support Record Growth and Upcoming Product Launches
Xtant Medical Completes Sale of its Coflex® Assets and Paradigm OUS Businesses to Companion Spine
BELGRADE, Mont., Dec. 1, 2025 /PRNewswire/ -- Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for spinal and other orthopedic conditions, today announced that the company has completed its previously announced sale of certain non-core Coflex® spinal implant assets and all OUS entities … [Read more...] about Xtant Medical Completes Sale of its Coflex® Assets and Paradigm OUS Businesses to Companion Spine
New System to Safely Remove Vertiflex Implants and Preserve the Spinous Processes — Launched by KIC Ventures
FORT LAUDERDALE, Fla., Dec. 1, 2025 /PRNewswire/ -- KIC Ventures, inventor of the global movement in LESS Exposure Spine Surgery (LESS™) and a leader in interventional spine surgery innovation, today announced the release of VertiFix, a breakthrough technology designed to address a critical and growing challenge facing interventional pain management (IPM) physicians: the safe … [Read more...] about New System to Safely Remove Vertiflex Implants and Preserve the Spinous Processes — Launched by KIC Ventures
Companion Spine LLC Completes Acquisition of the Business and Assets of Paradigm Spine GmbH, including the Coflex® and CoFix® Spine Implants and All Other Implant Systems, from Xtant™ Medical Holdings, Inc.
BORDEAUX, France & NEW YORK--(BUSINESS WIRE)--Companion Spine LLC (“Companion Spine” or “the Company”), the French-American specialist in spine implant surgery, announced today that it has completed its previously announced acquisition of the Coflex® Interlaminar Stabilization® device (“Coflex®”) and CoFix® Posterior MIS Fusion System (“CoFix®”) implants, Paradigm Spine … [Read more...] about Companion Spine LLC Completes Acquisition of the Business and Assets of Paradigm Spine GmbH, including the Coflex® and CoFix® Spine Implants and All Other Implant Systems, from Xtant™ Medical Holdings, Inc.
SINTX Technologies Signs Supply Agreement with EVONIK to Manufacture Silicon Nitride–PEEK Compound for AI-Assisted, 3D-Printed Patient-Specific Implants
SALT LAKE CITY, Utah, Dec. 01, 2025 (GLOBE NEWSWIRE) -- SINTX Technologies, Inc. (NASDAQ: SINT) (“SINTX” or the “Company”), an advanced ceramics and biomaterials company, today announced that it has signed a supply agreement with Evonik Corporation (“EVONIK”), a global leader in high‑performance polymers, to manufacture the Company’s proprietary silicon nitride–PEEK … [Read more...] about SINTX Technologies Signs Supply Agreement with EVONIK to Manufacture Silicon Nitride–PEEK Compound for AI-Assisted, 3D-Printed Patient-Specific Implants