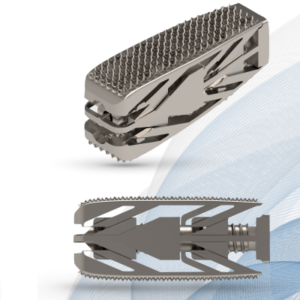
Modena, June 14th 2021 Tsunami Medical, among the leaders in Spine technology innovation, focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch Procida, 3D printed PLIF cage. Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second Generation of Spinal […]
NEWS
Spinal Elements® Announces Publication of Positive MIS TLIF Clinical Outcomes Data using the Luna® XD Multi-Expandable Interbody Implant
Carlsbad, CA, June 8, 2021 – Spinal Elements, a spine technology company, today announced the publication of positive clinical outcomes data with the Luna XD multi-expandable implant system. The retrospective analysis of 69 consecutive patients over a 2-year period demonstrated statistically significant improvement in leg pain, back pain, disability index, and additional patient-reported outcome measures. […]
Vuzix Smart Glasses Expand AR Surgical Product Presence to Support Shoulder, Knee and Spine Surgeries
ROCHESTER, N.Y., June 9, 2021 /PRNewswire/ — Vuzix® Corporation (NASDAQ: VUZI), (“Vuzix” or, the “Company”), a leading supplier of Smart Glasses and Augmented Reality (AR) technology and products, today announced the market expansion to support the NextAR surgical AR platform developed by Medacta International, one of the world’s largest providers of innovative orthopedic products focusing on healthcare sustainability. Having used the […]
SeaSpine Announces Limited Commercial Launch of the WaveForm® L (Lateral) 3D-Printed Interbody System
CARLSBAD, Calif., June 08, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch of its 3D-printed WaveForm L (Lateral) Interbody System. WaveForm L is designed for the LLIF (lateral lumbar interbody fusion) procedure, and […]
Surgalign Holdings Announces Collaboration with Inteneural Networks, a Leading Developer of Artificial Intelligence for Clinical Neurosciences
DEERFIELD, Ill., June 07, 2021 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (NASDAQ: SRGA), a global medical technology company focused on elevating the standard of care through the evolution of digital surgery, and Inteneural Networks Inc., a developer of innovative artificial intelligence (AI) based applications focused on fully autonomous analytics of central nervous system imaging, today […]
ATEC Welcomes EOS Imaging Team
CARLSBAD, Calif., June 07, 2021 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (Nasdaq: ATEC), is a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery. Centered on a singular vision to better inform surgery, ATEC today welcomes the EOS imaging team in a combination forged to improve the predictability of the patient experience […]
Tsunami Medical Announces the Launch of Ustica, 3D Printed TLIF Titanium Cage with Expansion Feature
Modena, June 3, 2021–Tsunami Medical, among the leaders in Spine technology innovation, focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch of Ustica, 3D printed TLIF cage. Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second Generation of Spinal […]
UPDATED: +117 Cervical Cages to Know!
We have finally finished updating the Cervical Interbody Cages (ACIF) section. Last week we published the part related to Stand-alone cages and today we have also incorporated 3D titanium cages. From today, the list of 117 cervical cages is at your disposal, incorporating (in many of them) the Videos or Brochures that are available. We […]
Tsunami Medical Announces the Launch of Ortigia-J and Caprera, 3D Printed OLIF Cages with Built-in Fixation and Expansion Features
Modena, June 4th 2021–Tsunami Medical, among the leaders in Spine technology innovation, focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch of two additional 3D printed antero-lateral OLIF cage solutions. Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second […]
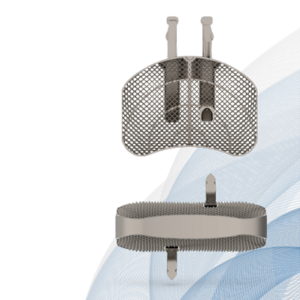
Globus Medical Launches CORBEL™ Lateral ALIF Interbody Spacer
Introducing CORBEL™, Globus Medical’s solution for lateral position ALIF surgery! This system features in-line integrated anchor technology to reduce the number of procedural steps compared to screws, offset instruments to simplify midline implant placement, and SintrOS™ surface technology designed to encourage cellular activity at the bone interface. CORBEL™ requires supplemental fixation. Features and Benefits SintrOS™ […]
Medtronic Gains FDA Clearance of UNiD™ Patient-Specific Rods for Use with CD Horizon™ Solera™ Voyager™ and Infinity™ OCT Spinal Systems
DUBLIN, June 3, 2021 /PRNewswire/ — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. Food and Drug Administration (FDA) clearance of patient-specific UNiD™ Rods for use with Medtronic CD Horizon™ Solera™ Voyager™ and Infinity™ OCT spinal systems, expanding the utility of the company’s UNiD™ Adaptative Spine Intelligence (ASI) technology. UNiD Rods are […]
Spectrum Announces Acquisition of Unique Surface Technology for Spinal Trauma
ATLANTA, June 2, 2021 /PRNewswire/ — Spectrum Spine, spinal device supplier and leader in the development of spinal surgical device inventions, announced that it has acquired a highly unique and proprietary surface technology for spinal trauma and has begun operations with the device. BIOBraille™ is a state-of-the-art surface technology with 14 related patents, exclusively licensed to Spectrum […]
Empirical Spine Closes $10 Million Series B Financing
SAN CARLOS, Calif., June 2, 2021 /PRNewswire/ — Empirical Spine, Inc., maker of the LimiFlex Paraspinous Tension Band, today announced the closing of a Series B financing of $10 million. SHD (Scientific Health Development) led the round, with additional investment from GP&G (Green Park & Golf) and other syndicate members. The funds will be used to advance the product on […]
Signature of a Memorandum of Understanding for the acquisition of the Distimp start-up
Ecully, 1 June 2021 – 5 :45 p.m.—Spineway, specialized in innovative implants for the treatment of severe disorders of the spinal column (spine), signed a Memorandum of Understanding for the acquisition of 100% of the capital –subject to conditions precedent being met – of Distimp, a European start-up with which the Group began exclusive negotiations last March. Distimp, based in Nîmes, is specialized in the design, manufacture and sale of implants and instruments […]
OrthoPediatrics Corp. Launches RESPONSE™ Neuromuscular Scoliosis System
WARSAW, Ind., May 28, 2021 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics”) (Nasdaq: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, announced the launch of its RESPONSE™ Neuromuscular Scoliosis System. This will be the 36th surgical system the Company has launched. The system received FDA 510(k) clearance in 2020, and the Company has been preparing […]
Tsunami Medical announces the launch of Alicudi-J, 3D Printed ALIF Titanium Cage with Built-in Fixation Mechanism
Modena, May 27th 2021–Tsunami Medical, among the leaders in Spine technology innovation focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch of an additional 3D printed ALIF cage solution. Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second Generation […]
Medtronic Reports Fourth Quarter and Fiscal Year 2021 Financial Results; Announces 9% Dividend Increase
DUBLIN, May 27, 2021 /PRNewswire/ — Medtronic plc (NYSE:MDT) today announced financial results for its fourth quarter and fiscal year 2021, which ended April 30, 2021. The company reported fourth quarter worldwide revenue of $8.188 billion, an increase of 37 percent as reported and 32 percent on an organic basis, which adjusts for the $241 million benefit of foreign currency translation. The […]
Safe Orthopaedics announces CE mark for new Hickory screw
Éragny-sur-Oise, France, May 27th, 2021 at 8:55 a.m. CET – Safe Orthopaedics (FR0013467123 – ALSAF), a company specialising in the design, manufacture and marketing of ready-to-use technologies for back surgery, with a particular focus on the safety of emergency vertebral fractures, announced today that it has obtained CE Mark approval for Hickory, a new treatment for the management […]
Spinal Stabilization Technologies Ltd Earns CE Mark, FDA “Breakthrough Designation” For PerQdisc Nucleus Replacement System For Degenerative Disc Disease
KILKENNY, Ireland, May 25, 2021 /PRNewswire/ — Spinal Stabilization Technologies, Ltd. (SST), Kilkenny, Ireland announced today that it has earned the CE Mark and the FDA’s “breakthrough designation” for its PerQdisc™ Nucleus Replacement System. PerQdisc is the only commercially available lumbar nucleus replacement system in the world. The PerQdisc device replaces the nucleus pulposus of the intervertebral disc in the L1 […]
Tsunami Medical announces launch of Stromboli-J and Giannutri,3D printed DLIF cages with built-in fixation and expansion features
Modena, May 21st 2021–Tsunami Medical, among the leaders in Spine technology innovation, focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch of two additional 3D printed DLIF cage solutions. Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a Second Generation […]
Tsunami Medical Announces the Launch of Capri-Z, 3D Printed ACIF Cage with Built-in Fixation
Modena, May 19th 2021–Tsunami Medical, among the leaders in Spine technology innovation, focusing on additive manufactured solutions for spine surgery and diagnostic invasive procedures, today announces the launch of Capri-Z: a 3D printed ACIF cage with built-in fixation mechanism.Years of cautious research and dedication by Tsunami Medical’s team have culminated with the achievement of a […]
CUREXO’s spinal surgery robot, ‘CUVIS-spine’ acquired FDA (US) licensing.
SEOUL, South Korea, May 21, 2021 /PRNewswire/ — On the 20th, the Medical robot specialist company CUREXO Corp. (060280) announced their independently developed spinal surgery robot ‘CUVIS-spine’ has acquired FDA (US) licensing. This FDA (US) license, followed by Korea (2019) and Europe CE (2020) certificate is the 3rd license that they have acquired and now their product can […]
15 Cervical Cages to know Today! Soon, many more … up to 100!
Dear visitors and followers, Today we bring you 15 different cervical cages. Some of them are well known and others not so much, but all of them are aligned with the most innovative designs or materials. These days we are updating our ACIF section to be able to present you in the next few days […]
ulrich medical USA® Announces Exceptional First Quarter Sales
ST. LOUIS, May 21, 2021 /PRNewswire/ — ulrich medical USA, Inc., a medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, reported that the first quarter of 2021 marked the strongest quarterly revenue in the company’s history. Despite the challenges presented by COVID, monthly sales have set a new bar for ulrich medical USA. In […]
SeaSpine Announces Closing of 7D Surgical Acquisition
CARLSBAD, Calif., May 20, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the closing of the previously announced acquisition of 7D Surgical. 7D Surgical is a Toronto-based company that develops advanced optical technologies and machine vision-based registration […]
SpineVision announces acquisition of Global S
SpineVision is proud to announce the acquisition of Global S, a privately held Lyon-based company. Global S is a distributor specialized in spine surgery products that has a strong footprint in France and Switzerland. This acquisition will consolidate SpineVision’s leadership position in France and bring new opportunities for Swiss market development. To reinforce this great […]
NuVasive Appoints Daniel J. Wolterman as Independent Board Chair
SAN DIEGO, May 19, 2021 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, announced today the appointment of Daniel J. Wolterman as independent chair of the NuVasive Board of Directors, effective immediately. Mr. Wolterman succeeds Gregory T. Lucier as the Company’s Board chair, following Mr. Lucier’s retirement from […]