Over the past five years, Barry served as the CEO of Nuvasive, a spine specialist company, which was acquired by the orthopedic company Globus Medical in September. Prior to his tenure at Nuvasive, Barry held leadership positions at Medtronic and Covidien. In March, Barry will join 3M to lead its Medical Solutions Division, encompassing wound care products. His … [Read more...] about 3M Appoints Former NuVasive CEO as President of Medical Solutions
NEWS
Orthofix Announces Commercial Partnership with MRIguidance
LEWISVILLE, TEXAS–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced it has entered into an agreement with MRIguidance to distribute its BoneMRI™ imaging software in the U.S. The Company also announced the successful completion of the world’s first eight cases utilizing BoneMRI software … [Read more...] about Orthofix Announces Commercial Partnership with MRIguidance
ChoiceSpine LLC Announces Changes in Leadership Roles
KNOXVILLE, Tenn.--(BUSINESS WIRE)--ChoiceSpine LLC, a medical device company in Knoxville, Tennessee, and privately held by Altus Capital Partners, announces that Marty Altshuler and Rick Henson, founders and Co-Presidents of ChoiceSpine, transitioned to Executive Advisor roles following 18 years of leading the company. Conjointly, effective January 01, 2024, the Board of … [Read more...] about ChoiceSpine LLC Announces Changes in Leadership Roles
Centinel Spine® Concludes Transformative Year with Record prodisc® Total Disc Replacement Revenue and Growth in December and Fourth Quarter 2023
WEST CHESTER, Pa., Jan. 18, 2024 /PRNewswire/ -- Centinel Spine®, LLC, ("the Company") a leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced achievement of record year-end 2023 … [Read more...] about Centinel Spine® Concludes Transformative Year with Record prodisc® Total Disc Replacement Revenue and Growth in December and Fourth Quarter 2023
Accelus Announces Closing of $20 Million Debt Facility with Symbiotic Capital
PALM BEACH GARDENS, Fla., Jan. 18, 2024 (GLOBE NEWSWIRE) -- Accelus, a privately held medical technology company committed to becoming the global market leader in expandable spinal implant technologies, announced today that it has received a significant financial investment through a $20 million debt facility from Symbiotic Capital. This strategic funding from Symbiotic … [Read more...] about Accelus Announces Closing of $20 Million Debt Facility with Symbiotic Capital
We are proud to announce that GS Medical USA will be Sponsor of SPINEMarketGroup in 2024!
Thank you GS Medical USA! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2024 through your PLATINUM sponsorship. About GS Medical USA A leader in the surgical spine industry, GS Medical is a supplier of spinal implants and instrumentation and a provider of high-quality surgical solutions. Our mission is simple: improve the … [Read more...] about We are proud to announce that GS Medical USA will be Sponsor of SPINEMarketGroup in 2024!
Bolt® Navigation Announces Closing of its Series B Financing
CONCORD, Mass., Jan. 17, 2024 /PRNewswire/ -- Bolt Navigation, a privately-held leading developer of Handheld Surgical Navigation systems, announced today that it has closed its Series B financing. The round was once again led by Grand Oaks Capital, the investment group for the family office of Paychex founder Tom Golisano, with participation … [Read more...] about Bolt® Navigation Announces Closing of its Series B Financing
We are proud to announce that Viktor Hegedüs GmbH will be Sponsor of SPINEMarketGroup in 2024!
Thank you Viktor Hegedüs GmbH! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2024 through your PLATINUM sponsorship. About Viktor Hegedüs GmbH The name Viktor Hegedüs has stood for extraordinary innovations and high-quality precision in the fields of medical technology and industrial applications for many years … [Read more...] about We are proud to announce that Viktor Hegedüs GmbH will be Sponsor of SPINEMarketGroup in 2024!
Amanda Bloom Joins Spineology as Executive Vice President of Marketing
ST. PAUL, Minn.--(BUSINESS WIRE)--Spineology Inc. ("Spineology" or the "Company"), the leader in ultra-minimally invasive spine surgery, announced today the addition of Amanda Bloom as Executive Vice President, Marketing. In this role, Bloom will lead the Product Marketing, Education, and Clinical organizations. Bloom comes to Spineology with extensive experience, having served … [Read more...] about Amanda Bloom Joins Spineology as Executive Vice President of Marketing
Med-Surgical Services, Inc. Hits CBYON Sales Milestone on Heels of $4 Million Series A Financing Round
San Diego, CA, Jan. 16, 2024 (GLOBE NEWSWIRE) -- Med-Surgical Services, Inc. (MSSI), the manufacturer of the pioneering CBYON Eclipse image-guided surgery (IGS) system, has surpassed its initial commercial goal by selling 25 navigation systems in its first year of service. This sales milestone comes on the heels of successfully completing a $3.9 million Series A funding round … [Read more...] about Med-Surgical Services, Inc. Hits CBYON Sales Milestone on Heels of $4 Million Series A Financing Round
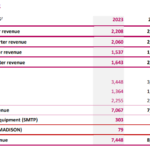
IMPLANET reports 2023 annual revenue of €7.4 million
Bordeaux, Boston, January 15, 2024 – 6:00 pm CET: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in implants for orthopedic surgery and the distribution of technological medical equipment, today announced its 2023 annual revenue and its cash position as of December 31, 2023. Ludovic … [Read more...] about IMPLANET reports 2023 annual revenue of €7.4 million
We are proud to announce that Normmed will be Sponsor of SPINEMarketGroup in 2024!
Thank you Normmed! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2024 through your PLATINUM sponsorship. About Normmed Normmed makes a difference in both domestic and international markets with innovative solutions in its field and progresses on this path. Normmed, which manufactures spinal implants and instruments and … [Read more...] about We are proud to announce that Normmed will be Sponsor of SPINEMarketGroup in 2024!
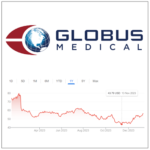
Do you have shares of Globus Medical? Unsure whether to sell or hold? Read this first!!
Numerous investors holding Globus Medical shares face this dilemma after almost a year of market fluctuations. The market journey, akin to a rollercoaster ride, began with the fusion of Globus Medical and NuVasive, which failed to spark enthusiasm on Wall Street, setting the stage for a complex interplay of market dynamics and leaving you pondering the fate of your investment.A … [Read more...] about Do you have shares of Globus Medical? Unsure whether to sell or hold? Read this first!!
The Future of ZimVie’s Spine Business Post-Sale: Exploring Potential Alternatives!
In a strategic move, ZimVie Inc. recognized globally for its leadership in the dental and spine markets, has finalized a definitive agreement to sell its spine business to H.I.G. Capital, a prominent global alternative investment firm. H.I.G. Capital, a global private equity firm, has a strong track record of successfully managing and growing businesses across various sectors. … [Read more...] about The Future of ZimVie’s Spine Business Post-Sale: Exploring Potential Alternatives!
We are proud to announce that GLOBAL biomedica will be Sponsor of SPINEMarketGroup in 2024!
Thank you GLOBAL biomedica! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2024 through your PLATINUM sponsorship. About GLOBAL biomedica GLOBAL biomedica is manufacturer of orthopedic titanium implants, extended by designing and constructing in collaboration with leading experts in this field, using innovative and high … [Read more...] about We are proud to announce that GLOBAL biomedica will be Sponsor of SPINEMarketGroup in 2024!
We are proud to announce that Tsunami Medical will be Sponsor of SPINEMarketGroup in 2024!
Thank you Tsunami Medical! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2024 through your PLATINUM sponsorship. About Tsunami Medical The company has been founded in 1997 as a subcontractor of some big manufacturing companies of invasive diagnostic devices. Over the years we bought the Bloodline trade mark, very well … [Read more...] about We are proud to announce that Tsunami Medical will be Sponsor of SPINEMarketGroup in 2024!
Globus Medical Reports Preliminary Record Fourth Quarter and Full Year Sales Results
AUDUBON, Pa., Jan. 10, 2024 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced preliminary unaudited sales results for the fourth quarter and full year ending December 31, 2023. The company anticipates fourth quarter 2023 sales of approximately $615.5 million, an increase of 124.2 percent over the fourth … [Read more...] about Globus Medical Reports Preliminary Record Fourth Quarter and Full Year Sales Results
3Spine Announces Completion of FDA Clinical Trial Enrollment Achieving 325 Surgeries in 2023
CHATTANOOGA, Tenn., Jan. 10, 2024 /PRNewswire/ -- 3Spine, Inc., a medical device company developing a transformational alternative to lumbar spinal fusion, today announced achievement of US clinical trial enrollment, with 151 completed MOTUS lumbar total joint replacement surgeries and 174 real-world posterior lumbar fusions as of December 2023. The company … [Read more...] about 3Spine Announces Completion of FDA Clinical Trial Enrollment Achieving 325 Surgeries in 2023
Providence Medical Technology Announces FDA Clearance of CAVUX® FFS-LX Lumbar Facet Fixation System for 1- and 2-Level Lumbar Spinal Fusion
PLEASANTON, Calif., Jan. 10, 2024 /PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Facet Fixation System for use in lumbar spinal fusion … [Read more...] about Providence Medical Technology Announces FDA Clearance of CAVUX® FFS-LX Lumbar Facet Fixation System for 1- and 2-Level Lumbar Spinal Fusion