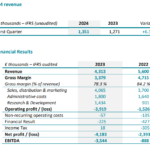
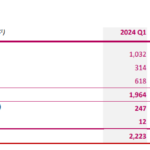
PARIS (France), BOULDER (CO, USA), April 17, 2024, - 06:00 pm CEST - SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) unique sensing technology using electrical conductivity local measurement in real time to secure and streamline the placement of bone implants, announced today its full-year 2023 financial results, for … [Read more...] about SpineGuard announces its full-year 2023 financial results and its first quarter 2024 sales
NEWS
Spineway: Growth of 28% of 2024 first quarter revenue
Spineway, a specialist in innovative implants for the treatment of severe spine disorders, recorded revenue of €3.1 million in the first quarter of 2024, an increase of 28% compared with the same period in 2023. This purely organic growth (with no scope effect) reflects the Group’s strong sales momentum in its two main regions, Europe and Latin America, which accounted for 85% … [Read more...] about Spineway: Growth of 28% of 2024 first quarter revenue
Orthofix Names Andres Cedron as New Chief Legal Officer
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced that Andres Cedron has joined the company as Chief Legal Officer. Cedron most recently served as Vice President and Corporate Secretary at Stryker Corporation, a $20B+ global medical device company. In his corporate officer position, Cedron … [Read more...] about Orthofix Names Andres Cedron as New Chief Legal Officer
Over 5,000 Procedures Completed in U.S. with Centinel Spine’s prodisc® C Vivo and prodisc C SK Cervical Total Disc Replacement System
WEST CHESTER, Pa., April 16, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the completion of the … [Read more...] about Over 5,000 Procedures Completed in U.S. with Centinel Spine’s prodisc® C Vivo and prodisc C SK Cervical Total Disc Replacement System
Captiva Spine Welcomes New Sales Leadership Erik Gottschalk, to the commercial team to spearhead the Growth of ASC-Ready product Portfolio
Jupiter, FL – April 2024 – Captiva Spine®, a medical technology organization connecting healthcare professionals, distribution specialists, and healthcare facilities with cutting-edge spinal care technology, proudly announces the appointment of Erik Gottschalk as the Director of Sales. With an impressive track record and extensive experience in the spinal device … [Read more...] about Captiva Spine Welcomes New Sales Leadership Erik Gottschalk, to the commercial team to spearhead the Growth of ASC-Ready product Portfolio
Xstim, Inc. Receives FDA Approval for Xstim™ Spine Fusion Stimulator
IRVING, Texas, April 16, 2024 /PRNewswire/ -- Xstim, Inc., a pioneering developer and manufacturer of cutting-edge bone growth stimulation systems, is thrilled to announce its recent Premarket Application (PMA) approval from the U.S. Food and Drug Administration (FDA) for Xstim™ Spine Fusion Stimulator. Engineered with patient comfort and convenience in mind, the Xstim™ Spine … [Read more...] about Xstim, Inc. Receives FDA Approval for Xstim™ Spine Fusion Stimulator
SpinePoint Medical Unveils Innovative Spinal Implant Solutions
Reno, NV, April 16, 2024 /PRNewswire-PRWeb/ -- SpinePoint Medical, a leading innovator in spine surgery technology, is proud to announce the upcoming launch of its groundbreaking solution aimed at revolutionizing spine care. With a commitment to advancing surgical techniques and improving patient outcomes, SpinePoint introduces a transformative approach that promises to … [Read more...] about SpinePoint Medical Unveils Innovative Spinal Implant Solutions
IMPLANET Reports its 2024 First-Quarter Revenue
Bordeaux, Boston, April 9, 2024 – 6:00 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral implants for orthopedic surgery and the distribution of technological medical equipment, today announces its revenue for the first quarter of 2024. Ludovic Lastennet, IMPLANET’s … [Read more...] about IMPLANET Reports its 2024 First-Quarter Revenue
Johnson&Johnson MedTech Partners with Cardiva for Distribution of DePuy Synthes Spine Products in Spain
Cardiva, a manufacturer and distributor of medical products, has entered into a partnership to distribute Johnson&Johnson MedTech's spine surgery products in Spain for five years. The agreement includes the national marketing of DePuy Synthes' Spine range.With this partnership, Cardiva aims to provide materials to simplify and make spinal surgical interventions less … [Read more...] about Johnson&Johnson MedTech Partners with Cardiva for Distribution of DePuy Synthes Spine Products in Spain
Proprio Announces Strategic Partnership with Biedermann to Revolutionize Surgery Through AI-Powered Guidance
SEATTLE, April 9, 2024 /PRNewswire/ -- Proprio, the leader in AI-powered surgical technology, today announced a landmark multi-phase partnership with the Biedermann Group, the prominent innovator in next generation spinal implant systems and procedural solutions. Initially, the companies will collaborate to integrate Biedermann's advanced spinal implants with Proprio's … [Read more...] about Proprio Announces Strategic Partnership with Biedermann to Revolutionize Surgery Through AI-Powered Guidance
DePuy Synthes will officially launch the TriALTIS™ Spine System, next generation pedicle screw system, at #IMAST2024
DePuy Synthes will officially launch their new system TriALTIS™ Spine System at #IMAST2024. About TriALTIS™ Spine System Combining a new portfolio of implants with a digital ecosystem, the TriALTIS™ Spine System aims to address unmet clinical needs and help surgeons achieve more consistent outcomes in treating complex spine conditions, inclusive of degenerative, tumor, … [Read more...] about DePuy Synthes will officially launch the TriALTIS™ Spine System, next generation pedicle screw system, at #IMAST2024
Hyprevention Becomes a U.S. Based Company, Focusing Commercial Sales Operations in the U.S.
NEW YORK--(BUSINESS WIRE)--Hyprevention Inc. ("Hyprevention"), a medtech company, announced today that it incorporated in Delaware, becoming the new parent company of the group previously established in France in 2010. Hyprevention develops and markets the Strutplasty® Technology. The Strutplasty® Technology is 510K cleared, under the trade name V-Strut© Transpedicular … [Read more...] about Hyprevention Becomes a U.S. Based Company, Focusing Commercial Sales Operations in the U.S.
Spineology® Appoints new Member to Leadership Team; Spine Veteran Emory Rooney joins as Executive Vice President of Sales
ST. PAUL, Minn.--(BUSINESS WIRE)--Spineology Inc. ("Spineology" or the "Company"), the leader in ultra-minimally invasive spine surgery, announced today the addition of Emory Rooney as Executive Vice President, Sales. In this role, Rooney will lead U.S. market growth and sales efforts. Rooney comes to Spineology with extensive spine sales experience, having served in Medical … [Read more...] about Spineology® Appoints new Member to Leadership Team; Spine Veteran Emory Rooney joins as Executive Vice President of Sales
From Germany to the World: Realists Expands Global Reach With NewDistribution Partnerships in Brazil and Japan
LEIPZIG, GERMANY, [April 2, 2024] – Realists Training Technologies GmbH, a leading provider of innovative surgical training solutions is proud to announce its expansion into Brazil and Japan through strategic partnerships with Innport – Innovative Imports (Innport Comércio de Distribuição de Produtos para Saúde LTDA) and Muranaka Medical Instruments Co. Ltd., respectively. … [Read more...] about From Germany to the World: Realists Expands Global Reach With NewDistribution Partnerships in Brazil and Japan
Captiva Spine® Introduces TransFasten® LSF, Lateral Si Fusion System, Creating a Comprehensive Selection of ASC-READY SI Fusion Solutions
Captiva Spine®, a medical technology organization connecting healthcare professionals, distribution specialists, and healthcare facilities with cutting-edge spinal care technology, announces the launch of the TransFasten-LSF Lateral SI (Sacroiliac) Fusion System. The addition of this system marks a significant advancement that builds upon the TransFasten® SI fusion product … [Read more...] about Captiva Spine® Introduces TransFasten® LSF, Lateral Si Fusion System, Creating a Comprehensive Selection of ASC-READY SI Fusion Solutions
H.I.G. Capital Acquires the Spine Business of ZimVie Rebranded as Highridge Medical
MIAMI--(BUSINESS WIRE)--H.I.G. Capital (“H.I.G.”), a leading global alternative investment firm with $60 billion of capital under management, is pleased to announce that one of its affiliates has completed the acquisition of the Spine division of ZimVie, Inc (“ZimVie”, NYSE: ZIMV). The acquired business will operate as an independent entity and has been renamed Highridge … [Read more...] about H.I.G. Capital Acquires the Spine Business of ZimVie Rebranded as Highridge Medical
NGMedical Celebrates One-Year Anniversary of the New Headquarter in Germany
NONNWEILER, SAARLAND, GERMANY, March 28, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating innovative technologies for spinal application is proud to celebrate the one-year anniversary of its state-of-the-art headquarter located in Nonnweiler, Germany. This milestone marks a year of innovation and growth for the … [Read more...] about NGMedical Celebrates One-Year Anniversary of the New Headquarter in Germany
PathKeeper Surgical enters into know-how agreement with Mayo Clinic for low-radiation pediatric spine surgery
KFAR SABA, Israel, March 25, 2024 /PRNewswire/ -- PathKeeper Surgical, an Israeli medical technology company committed to advancing surgical solutions through innovative camera and machine learning technologies, announced it has entered into a know-how agreement with Mayo Clinic to research radiation levels in pediatric spine surgeries. The research will … [Read more...] about PathKeeper Surgical enters into know-how agreement with Mayo Clinic for low-radiation pediatric spine surgery
Centinel Spine® to Sponsor Major Worldwide Live Webinar Symposium on Cervical Disc Arthroplasty
WEST CHESTER, Pa., March 21, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced that it will sponsor an upcoming major … [Read more...] about Centinel Spine® to Sponsor Major Worldwide Live Webinar Symposium on Cervical Disc Arthroplasty