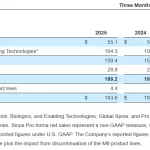
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a leading global medical technology company, today reported its financial results for the first quarter ended March 31, 2025, updated its full-year 2025 net sales guidance, and reaffirmed its full-year 2025 non-GAAP adjusted EBITDA and positive free cash flow guidance. All pro forma measures contained … [Read more...] about Orthofix Reports First Quarter 2025 Financial Results
NEWS
Curiteva & Promimic – Bonded by Innovation
HUNTSVILLE, Ala., May 6, 2025 (Newswire.com) - Curiteva, a leader in 3D-printed Trabecular PEEK implant technology, is pleased to announce an expanded partnership with Promimic, pioneers in nanotechnology, delivering advanced implant solutions with unmatched capabilities. The partnership combines Promimic's groundbreaking surface modification with Curiteva's innovative … [Read more...] about Curiteva & Promimic – Bonded by Innovation
J&J’s Spine Business—Stability Amid Uncertainty?
The latest results from Johnson & Johnson’s “Spine, Sports & Other” segment raise more questions than answers, especially for those of us closely watching the spine market.Over the last five quarters, the business has shown little change, with no clear growth trend. In fact, it ended Q1 2025 with the same sales figure as Q1 2024—$752 million. On the surface, that might … [Read more...] about J&J’s Spine Business—Stability Amid Uncertainty?
ATEC Reports First Quarter 2025 Financial Results and Raises Full-Year Guidance
CARLSBAD, Calif.--(BUSINESS WIRE)--Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended March 31, 2025, and recent corporate highlights. Recent Highlights “ATEC exists to revolutionize spine surgery, and our Q1 results show that our … [Read more...] about ATEC Reports First Quarter 2025 Financial Results and Raises Full-Year Guidance
No Sales, No Company — It’s That Simple!
This morning I came across an article on LinkedIn that I found genuinely insightful ( The Back Office Reality of Sales Compensation by Mike Kosikas ). It offered a well-structured explanation of how commercial compensation plans are built — the logic behind protecting margins, evolving incentive schemes, and balancing budgets. It made solid points and reflected the challenges … [Read more...] about No Sales, No Company — It’s That Simple!
Expanding Innovations™ Announces Record Growth for X-PAC® Expandable Cages
MOUNTAIN VIEW, Calif., May 1, 2025 /PRNewswire/ -- Expanding Innovations™ (EI), an emerging leader in expandable implant technology for spine surgery, is proud to announce it achieved record growth in Q1 2025. Revenue for X-PAC® Expandable Interbody Cages grew by an impressive 22% in comparison to the prior quarter, a testament to the growing confidence of … [Read more...] about Expanding Innovations™ Announces Record Growth for X-PAC® Expandable Cages
ComboSpine Appoints Spine Veteran Craig Corrance as Chief Executive Officer
Bethesda, Maryland Apr 29, 2025 (Issuewire.com) - ComboSpine™, a medical device company reshaping spinal fusion procedures with its innovative DEX360™ disc preparation and implant system, today announced the appointment of Craig Corrance as Chief Executive Officer. Corrance brings over three decades of experience in the global orthopedic device industry, with the last 20 years … [Read more...] about ComboSpine Appoints Spine Veteran Craig Corrance as Chief Executive Officer
Presentation at CFA Congress of Positive Results Obtained for SpineGuard’s New Ultrasound Technology
PARIS, and BOULDER (CO), April 29, 2025 - 6:00 pm CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, today announces that a team of researchers presented on the podium, a new scientific paper at the French Acoustic Congress (CFA) on April … [Read more...] about Presentation at CFA Congress of Positive Results Obtained for SpineGuard’s New Ultrasound Technology
Centinel Spine® prodisc® Total Disc Replacement Grows 33% Worldwide in First Quarter 2025, Surpassing $100M in Trailing Twelve Months Revenue
WEST CHESTER, Pa., April 29, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced achievement of nearly $28 … [Read more...] about Centinel Spine® prodisc® Total Disc Replacement Grows 33% Worldwide in First Quarter 2025, Surpassing $100M in Trailing Twelve Months Revenue
Dispute Over Spinal Implant Royalties Between British Surgeons and Johnson & Johnson
Dr. John Webb, a distinguished British spine surgeon and former physician to the Royal Family, is at the center of a legal dispute with U.S. medical giant Johnson & Johnson. Now aged 82, Webb is seeking compensation he says is rightfully owed for his role in developing groundbreaking spinal implants. According to a report by the Daily Mail, the dispute traces back to the … [Read more...] about Dispute Over Spinal Implant Royalties Between British Surgeons and Johnson & Johnson
IMPLANET to Co-Exhibit its Innovative Spine Solutions Alongside 8i Robotics’ Next-Gen Robotic Solutions at AANS in Boston
BORDEAUX, France & BOSTON--(BUSINESS WIRE)--Regulatory News: At this year’s American Association of Neurological Surgeons Annual Meeting (AANS) Annual Meeting in Boston, USA, IMPLANET (Paris:ALIMP), a leading global innovator in spine and medical technologies, will co-exhibit at booth #1029 alongside fellow innovator 8i Robotics, pioneering the development of a multi-arm … [Read more...] about IMPLANET to Co-Exhibit its Innovative Spine Solutions Alongside 8i Robotics’ Next-Gen Robotic Solutions at AANS in Boston
Tyber, Intech & Resolve Finalize Merger to Form Global CDMO+ for Musculoskeletal Device Solutions
New York, NY, April 24, 2025 - In a pivotal move shaping the future of MedTech, Tyber Medical, Intech, and Resolve Surgical Technologies have merged into a unified, leading developer, designer and manufacturer of surgical devices focused on serving the needs of MedTech OEMs and patients. The new group combines world-class implant and … [Read more...] about Tyber, Intech & Resolve Finalize Merger to Form Global CDMO+ for Musculoskeletal Device Solutions
CGBIO Receives FDA IDE Approval for NOVOSIS PUTTY, Advancing Toward U.S. Market Entry
SEOUL, South Korea — CGBIO(CEO Hyun Seung Yu), a leading Korean company specializing in bio-regenerative medicine, proudly announces that its innovative bone graft substitute, NOVOSIS PUTTY, has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA). This pivotal approval paves the way for a clinical trial in spinal fusion … [Read more...] about CGBIO Receives FDA IDE Approval for NOVOSIS PUTTY, Advancing Toward U.S. Market Entry
LEM Surgical Announces FDA Clearance of Dynamis Robotic Surgical System
BERN, Switzerland, April 24, 2025 (Newswire.com) - LEM Surgical, a developer of advanced robotic technologies for hard tissue surgery, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Dynamis Robotic Surgical System, marking a significant milestone in the evolution of robotic-assisted hard tissue surgery. The … [Read more...] about LEM Surgical Announces FDA Clearance of Dynamis Robotic Surgical System
Novadip reports key nonclinical and interim clinical data for NVDX3, its off-the-shelf allogenic product, in spine fusion
Mont Saint-Guibert, Belgium, April 22, 2025 – Novadip Biosciences, a late-stage clinical biotechnology company specializing in regenerative medicine, today announces positive results from its NVDX3 program, an allogenic bone grafting material created from human osteogenic adipose tissue-derived mesenchymal stem cells (ASCs). NVDX3 performed well in an animal model with … [Read more...] about Novadip reports key nonclinical and interim clinical data for NVDX3, its off-the-shelf allogenic product, in spine fusion
Xtant Medical Launches Trivium™ Advanced Bone Graft for Superior Performance
BELGRADE, Mont., April 23, 2025 /PRNewswire/ -- Xtant Medical Holdings, Inc.(NYSE American:XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal, orthopedic and woundcare disorders, is proud to announce the launch of Trivium™, a premium, next-generation demineralized bone matrix (DBM) allograft designed to elevate … [Read more...] about Xtant Medical Launches Trivium™ Advanced Bone Graft for Superior Performance
Captiva Spine’s WatchTower Spine Navigation Platform Enhancements Accelerate Clinical Adoption
The WatchTower Spine Navigation System is designed to be uncomplicated, compact, and cost-effective. The Navigation Platform empowers surgeons with CT-based accuracy and streamlined workflows, eliminating the need for intraoperative CT scans. JUPITER, Fla., April 23, 2025 /PRNewswire-PRWeb/ -- Captiva Spine, a privately held medical device company specializing in spinal … [Read more...] about Captiva Spine’s WatchTower Spine Navigation Platform Enhancements Accelerate Clinical Adoption
Augmedics Completes 10,000th Augmented Reality Spine Surgery With xvision Spine System
CHICAGO--(BUSINESS WIRE)--Augmedics, a pioneer in augmented reality (AR) surgical navigation, today announced it has treated 10,000 patients with the xvision Spine System®. The landmark achievement marks a new record for the use of augmented reality navigation for spine surgery. “Since inception, Augmedics has been a company of firsts – the first FDA-cleared AR navigation … [Read more...] about Augmedics Completes 10,000th Augmented Reality Spine Surgery With xvision Spine System
ATEC Launches PTP™ Corpectomy, The Next Evolution of Lateral Approach Surgery
CARLSBAD, Calif.--(BUSINESS WIRE)--Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced the commercial launch of its Prone TransPsoas (PTP™) Corpectomy system. This milestone marks the continued evolution of ATEC’s PTP surgical approach, expanding its capabilities to include … [Read more...] about ATEC Launches PTP™ Corpectomy, The Next Evolution of Lateral Approach Surgery