DALLAS, Feb. 25, 2026 /PRNewswire/ -- 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary TRUSS Implant Technology™, announced that it has received 510(k) clearance to market its SI Joint Truss System™. 4WEB's SI Joint Truss System is indicated for fusion of the sacroiliac joint for sacroiliac joint … [Read more...] about 4WEB Medical Receives 510(k) Clearance to Market its New SI Joint Truss System™
FDA APPROVED
Spine Innovation Announces FDA 510(k) Clearance for LOGIC™ Titanium Expandable Interbody System
CORONADO, Calif., Jan. 26, 2026 /PRNewswire/ -- Spine Innovation, LLC, a medical device startup that develops novel interbody fusion implants, announced today that is has received FDA 510(k) clearance to market the LOGIC™ Titanium Implant System. The LOGIC™ Implant System is the next generation LOGIC™ implant incorporating OsteoSync™ Ti, a patented, pure titanium … [Read more...] about Spine Innovation Announces FDA 510(k) Clearance for LOGIC™ Titanium Expandable Interbody System
FDA grants 510k approval for Lincotek’s Medical Division new Cervical Implant System
Lincotek’s SpineLinc to reduce time to market for orthopedic manufacturers. Parma, Italy | January 13, 2026: The Medical Division of Lincotek – global solution provider for services in the medical devices industry – has announced that the U.S. Food and Drug Administration (FDA) has granted 510k clearance for the company’s SpineLinc Anterior Cervical Implant … [Read more...] about FDA grants 510k approval for Lincotek’s Medical Division new Cervical Implant System
Vy Spine Announces FDA Clearance for VyBrate VBR System
Bountiful, UT – January 13, 2025—Vy Spine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its VyBrate VBR System which is designed for use in both the cervical and thoracolumbar spine for vertebral body replacement surgery. The VyBrate VBR is an … [Read more...] about Vy Spine Announces FDA Clearance for VyBrate VBR System
Spinal Simplicity Announces FDA Clearance of Minuteman® G6 MIS Fusion Device
Spinal Simplicity has received FDA 510(k) clearance for the Minuteman G6 MIS Fusion Device, a next-generation, minimally invasive platform designed to enhance procedural efficiency while preserving anatomy through a tissue-sparing, direct access approach. The Minuteman G6 expands the company's portfolio for aging spine care, with a phased U.S. commercial launch planned for Q1 … [Read more...] about Spinal Simplicity Announces FDA Clearance of Minuteman® G6 MIS Fusion Device
Companion Spine Announces FDA Premarket Approval (“PMA”) of the DIAM™ Spinal Stabilization System for the Treatment of Degenerative Disc Disease (“DDD”)
DIAM™ Spinal Stabilization System is the first posterior motion-preserving device approved in the U.S. for the treatment of moderate to severe primary low back pain A breakthrough treatment addressing both undertreatment and overtreatment in the current care pathway, with robust evidence demonstrating superiority over non-operative care DIAM™ Spinal Stabilization System … [Read more...] about Companion Spine Announces FDA Premarket Approval (“PMA”) of the DIAM™ Spinal Stabilization System for the Treatment of Degenerative Disc Disease (“DDD”)
Gad Medical Ltd. Receives Regulatory Approval to Start Sales of Ruthless Spine’s RJB Interoperative Angle Measurement Instrument in Israel, Marking Significant Expansion Following NASS Momentum
LOS ANGELES--(BUSINESS WIRE)--Ruthless Spine today announced that Gad Medical Ltd. has received regulatory approval to immediately start sales in Israel of Ruthless Spine’s RJB interoperative angle measurement instrument. This development follows their agreement on distribution rights to the RJB, enabling hospitals and surgical centers to access a U.S.-engineered implant … [Read more...] about Gad Medical Ltd. Receives Regulatory Approval to Start Sales of Ruthless Spine’s RJB Interoperative Angle Measurement Instrument in Israel, Marking Significant Expansion Following NASS Momentum
FFX® Receives FDA Clearance for Cervical Facet Arthrodesis, Expanding its U.S. indications
Strasbourg, France – December 8, 2025 – SC Medica today announced that its FFX® Facet FiXation System has received U.S. Food and Drug Administration (FDA) clearance for cervical use, marking a significant expansion of its clinical indications in the United States. Following the recent FDA authorization of FFX® for standalone lumbar facet … [Read more...] about FFX® Receives FDA Clearance for Cervical Facet Arthrodesis, Expanding its U.S. indications
SpineGuard Obtains the Clearance of Three New PediGuard® Models in China
PARIS and BOULDER (CO), December 4, 2025 – 6:00 pm CET - SpineGuard (FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced that the Chinese authorities (NMPA) have cleared the Curved and XS PediGuard models, as well as a … [Read more...] about SpineGuard Obtains the Clearance of Three New PediGuard® Models in China
EXALTA Group Receives FDA 510(k) Clearance for New Anterior Cervical Plating System
MARQUETTE, MI, UNITED STATES, November 13, 2025 /EINPresswire.com/ -- EXALTA Group, a next-generation partner to MedTech innovators, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Anterior Cervical Plating (ACP) System, designed to support stability and fusion in the cervical spine. The ACP system is indicated for … [Read more...] about EXALTA Group Receives FDA 510(k) Clearance for New Anterior Cervical Plating System
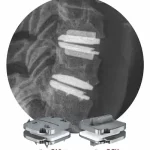
icotec Receives FDA Clearance for the CMORE® CT System, Expanding its BlackArmor® Engineered Carbon/PEEK Technology to the Posterior Cervicothoracic Spine
ALTSTÄTTEN, Switzerland and BOSTON, Nov. 13, 2025 /PRNewswire/ -- icotec, the pioneer of implantable devices made from BlackArmor® Engineered Carbon/PEEK, today announced that the U.S. Food and Drug Administration (FDA) has cleared the CMORE® CT System for use in the cervicothoracic spine. The CMORE® (CT) System is an enhanced set of … [Read more...] about icotec Receives FDA Clearance for the CMORE® CT System, Expanding its BlackArmor® Engineered Carbon/PEEK Technology to the Posterior Cervicothoracic Spine
Eminent Spine’s 3D Printed Titanium Posterior SI System Receives FDA 510(k) Clearance
Eminent Spine's 3D Printed Titanium posterior SI system compresses and transfixes the SI joint. Plano, TX, November 12, 2025 --(PR.com)-- Eminent Spine, a leader in spinal implant innovation, proudly announces that its revolutionary 3D Printed Titanium Posterior SI System has received FDA 510(k) clearance as of October 7, 2025. Innovative Design & Superior … [Read more...] about Eminent Spine’s 3D Printed Titanium Posterior SI System Receives FDA 510(k) Clearance
Captiva Spine Announces FDA Clearance for Flat-Panel C-Arm Image Calibrators and AI-Assisted Registration for WatchTower Spine Navigation, Advancing Efficiency, Radiation Reduction, and Future-Ready Access
Today’s facilities demand enabling technologies that integrate seamlessly into existing workflows without driving up costs or requiring new infrastructure. The WatchTower Spine Navigation System was designed around this philosophy: simple setup, familiar imaging, and precise guidance without workflow disruption. JUPITER, FL – November 2025 — In an era when technology is … [Read more...] about Captiva Spine Announces FDA Clearance for Flat-Panel C-Arm Image Calibrators and AI-Assisted Registration for WatchTower Spine Navigation, Advancing Efficiency, Radiation Reduction, and Future-Ready Access
Augmedics Announces FDA Clearance of Its Next-Generation AR Headset, X2™
CHICAGO--(BUSINESS WIRE)--Augmedics, a pioneer in augmented reality (AR) surgical navigation, today announced FDA 510(k) clearance of X2™, its next-generation AR headset for use with the xvision Spine System®. Unlike consumer-grade AR headsets intended for educational or entertainment use, the proprietary X2 headset is purpose built for surgery. The all-new form factor is … [Read more...] about Augmedics Announces FDA Clearance of Its Next-Generation AR Headset, X2™
FFX® Becomes the First Facet Cage Cleared by the FDA for Standalone Use.
Strasbourg, France – October 16, 2025 – SC Medica today announced that its FFX® Facet FiXation System has received U.S. Food and Drug Administration (FDA) clearance for standalone use, making it the first and only facet fusion cage approved for lumbar spine surgery without supplemental fixation. Following its initial FDA clearance in … [Read more...] about FFX® Becomes the First Facet Cage Cleared by the FDA for Standalone Use.
EXALTA Secures EU-MDR Certification for Titanium Compression Screws, Expanding Access to Advanced Trauma Solutions
BETHLEHEM, PA, UNITED STATES, October 15, 2025 /EINPresswire.com/ -- EXALTA Group, a global leader in the development and manufacturing of integrated OEM solutions for mission-critical medical devices, today announced it has obtained European Medical Device Regulation (EU-MDR) certification for its full range of headed and headless titanium compression screws used in trauma and … [Read more...] about EXALTA Secures EU-MDR Certification for Titanium Compression Screws, Expanding Access to Advanced Trauma Solutions
Centinel Spine® Receives Two-Level FDA Approval for prodisc® C Vivo and prodisc® C SK Match-the-Disc™ Cervical Total Disc Replacement Devices
WEST CHESTER, Pa., Oct. 14, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced U.S. Food and Drug Administration … [Read more...] about Centinel Spine® Receives Two-Level FDA Approval for prodisc® C Vivo and prodisc® C SK Match-the-Disc™ Cervical Total Disc Replacement Devices
Next-Generation AI-Driven SyncAR® Spine Receives FDA Clearance for Spine Surgery
By bringing MRI and CT directly into the operating room, SyncAR Spine turns preoperative planning imaging into real-time intraoperative guidance. CLEVELAND, Oct. 10, 2025 /PRNewswire/ -- Surgical Theater, the leader in surgical XR visualization, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for SyncAR® Spine, the next-generation … [Read more...] about Next-Generation AI-Driven SyncAR® Spine Receives FDA Clearance for Spine Surgery
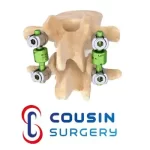
BDyn Posterior Dynamic Stabilization System Earns MDR Certification!
Cousin Surgery is proud to announce a major milestone: BDyn Posterior Dynamic Stabilization System has officially received MDR (Medical Device Regulation – EU 2017/745) certification! This achievement marks an important step forward in our ongoing commitment to the highest European standards of safety, quality, and performance for medical devices. Raising the bar for … [Read more...] about BDyn Posterior Dynamic Stabilization System Earns MDR Certification!