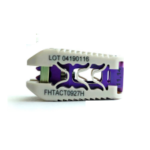
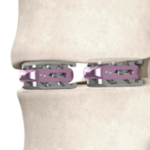
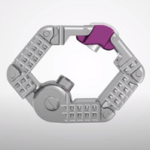
The dualX technology is comprised of a family of titanium expandable interbody devices designed to be implanted in lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) spinal procedures. The dualX portfolio contains varying footprints, heights and degrees of lordosis with post-expansion bone grafting … [Read more...] about dualX™ Dual Expanding Interbody Fusion System
PLIF/TLIF Expandable Cages
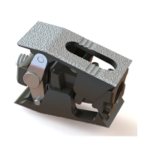
Explorer® TO Expandable Interbody System
Explorer® TO Expandable Interbody was designed to minimize impaction forces required with traditional static implants. The implant seamlessly expands within the disc space to restore height and lordosis optimized for each individual patient. The system consistently deliver autograft and/or allograft to fill any voids left in the implant from device expansion. With both a height … [Read more...] about Explorer® TO Expandable Interbody System
FlareHawk9™
FlareHawk9™ is a multidirectional expandable lumbar fusion device, featuring our innovative Adaptive Geometry™, that can be inserted at a low profile of 7mm or 9mm tall by 9mm wide before expanding up to 14mm tall and 14mm wide. Features: About Expandable Cages Expandable interbody cages allow surgeons to go through a small corridor and change the geometry of … [Read more...] about FlareHawk9™
FlareHawk7™
FlareHawk7™ is a multidirectional expandable lumbar fusion device, featuring Accelus’s innovative Adaptive Geometry™, that can be inserted at a low profile of 7mm tall by 7mm wide before expanding up to 12mm tall and 11mm wide. Its minimal insertion profile and instrumentation is design to respect the patient’s anatomy while facilitating the surgeon’s preferred … [Read more...] about FlareHawk7™
FORZA XP Expandable Spacer System
The FORZA® XP Expandable Spacer System offers titanium alloy expandable interbodies for Posterior Lumbar Interbody Fusion (PLIF) and Transforaminal Lumbar Interbody Fusion (TLIF) procedures. After insertion into the disc space, the interbodies can be expanded to fit the patient anatomy. Unlike many competitive expandables that trade off graft space in order to stay within the … [Read more...] about FORZA XP Expandable Spacer System
FLXfit15™
The FLXfit™ is an expandable, articulated interbody fusion device (IBFD) used in conjunction with supplemental fixation to provide structural stability in skeletally mature individuals following total or partial discectomy.The FLXfit™ is a unique titanium cage which articulates laterally as well as expands in height. This enables larger footprint support as well as … [Read more...] about FLXfit15™
IO™ Expandable Lumbar Interbody Fusion System
The IO™ Expandable Lumbar Interbody Fusion System is an expandable lumbar interbody fusion device used to provide structural stability in skeletally mature individuals following discectomy. It is indicated for intervertebral body fusion procedures in skeletally mature patients with degenerative disc disease (DDD) of the lumbar spine at one or two contiguous levels from … [Read more...] about IO™ Expandable Lumbar Interbody Fusion System
KLIMT™ Expandable Lumbar Interbody Fusion Cage
KLIMT™ Interbody Cages feature a multi-density and variable, textured surface on all sides, which is designed to enhance bone inter-digitation and capillary response. Collapsed cages are small enough to work through a small endoscopic access port. In addition, multiple footprints are available to accommodate a wide variety of patient anatomy and surgeon preferences. In … [Read more...] about KLIMT™ Expandable Lumbar Interbody Fusion Cage
LOGIC™ Implant System
The LOGIC™ Implant System is the next generation LOGIC™ implant incorporating OsteoSync™ Ti, a patented, pure titanium lattice material from Sites Medical which has been implanted in more than 250,000 patients since 2014. The patented LOGIC™ implant is designed with a reduced profile during implantation that more than doubles in size during expansion for maximum stability … [Read more...] about LOGIC™ Implant System
L-VARLOCK
L-VARLOCK® cage is a lumbar cage made of titanium, which makes it possible to perform posterior lumbar arthrodesis (PLIF) with restoration of disc height. Benefits: About KISCO International KISCO International has developed and manufactured medical devices for the spine since 1983. Today, it is a thriving company that has become an important player in … [Read more...] about L-VARLOCK
LorX® Expandable
LorX® Expandable PLIF Peek Cage has an unique design in various heights to restore the interbody space.Two adequate graft spaces for enhanced bone fusion.Specially designed (patent pending) expanding shaft has graft holes in the aim of filling bone graft after insertion. Benefits: Better Stability Anatomical Shape and Biocompatibility Easy … [Read more...] about LorX® Expandable
Luna 360 Interbody Fusion System
Luna XD allows an ALIF-sized, circular implant surgical delivery via a posterior, minimally-invasive access point to the intervertebral space. Once inserted, the Luna XD implant first expands horizontally to cover the disc footprint, creating a stable foundation. Then the implant expands vertically up to 16mm to restore disc height. The device includes up to 12° of lordosis to … [Read more...] about Luna 360 Interbody Fusion System
Leva® PX Interbody Device
The Leva™ Interbody Device is an expandable titanium implant that is optimized for ease of insertion and for maximum post expansion bone graft capability. The retractable bullet nose design facilitates insertion, while the instrumentation provides controlled expansion within the interbody space. Perhaps most important, following the implantation and expansion of the … [Read more...] about Leva® PX Interbody Device
Lucent XP Expandable
The Lucent XP Expandable Interbody device is a unique addition to the spinal fusion market. The main features of the device are its ability to expand in height and increase in lordotic angle after it has been surgically implanted.The height expansion helps restore the height of the spinal disc space and the lordotic angling helps correct the curvature of the … [Read more...] about Lucent XP Expandable
Omega XP Expandable Lumbar Interbody Device
The Omega XP Expandable Lumbar Interbody Device is designed for use in intervertebral body spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels from L2-S1. Features: About Spinal Elements Spinal Elements, headquartered in Carlsbad, CA, is a spine technology company for spine surgeons who demand … [Read more...] about Omega XP Expandable Lumbar Interbody Device
LATIS®
LATIS® is an innovative expandable lumbar interbody fusion spacer designed to provide an ALIF footprint through a TLIF approach. Features: About Globus Medical Globus Medical, Inc. is a leading spinal implant manufacturer and is driving significant technological advancements across a complete suite of spinal products. Founded in 2003, Globus’ single-minded … [Read more...] about LATIS®
Monaco
Monaco is a 3D printed expandable TLIF/PLIF Interbody Device. (This product is not cleared by the FDA for distribution in the U.S.) System Features About Neurostructures NeuroStructures is a medical device company focused on designing, developing, manufacturing and marketing proprietary, high-quality medical device systems. All of our products provide … [Read more...] about Monaco
MOJAVE™ PL 3D Expandable Interbody System
The MOJAVE™ PL 3D Expandable Interbody System is a fusion device designed to allow for independent control of the anterior and posterior height in the lumbar spine. Featuring infinite adjustment within the expansion range, the implant may be locked at any desired height and lordosis to aid in the restoration of sagittal balance. Designed with K2M’s Lamellar 3D … [Read more...] about MOJAVE™ PL 3D Expandable Interbody System
MLX
MLX is designed to be inserted through a conventional TLIF approach, MLX addresses the unique biomechanical loading and lordosis requirements of the lower lumbar spine by expanding to the dimensions of an ALIF graft, helping to optimize stability and maximize endplate coverage. Benefits: BIOMECHANICAL STABILITY DUAL FOOTPRINT OPTIONS: Large and … [Read more...] about MLX