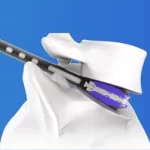
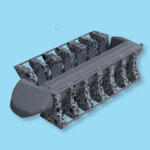
The "DIONYS PLEC (Posterior Lumbar Expandable Cage)" has an expandable shape and size after insertion of the cage, and can be fixed to fit more firmly. Features: About Expandable Cages Expandable interbody cages allow surgeons to go through a small corridor and change the geometry of the cage to correspond to what the patient’s spine requires. These implants … [Read more...] about DIONYS™ PLEC
EXPANDABLE CAGES
Elite™ Expandable Interbody Fusion System
The Elite™ Expandable Interbody Fusion System represents the next generation of expandable technology. Its unique design minimizes both neural retraction and insertion force, accommodates a larger graft channel than many competitive expandable cages, and provides controlled expansion to restore disc height, potentially providing indirect decompression. When paired with … [Read more...] about Elite™ Expandable Interbody Fusion System
ELEVATE™ Spinal System
The ELEVATE™ Spinal System consists of polyetheretherketone (PEEK) with tantalum markers and titanium expandable cages of various length and heights, which can be surgically implanted between two lumbar or lumbarsacral vertebral bodies to give support and correction during lumbar intervertebral body fusion. The hollow geometry of the implants allows them to be packed with … [Read more...] about ELEVATE™ Spinal System
eZspand™
eZspand™, the latest addition to Zavation’s portfolio, features unmatched expandable precision paired with continual expansion to provide an optimized fit for each patient. This optimized fit allows for enhancement of structural stability and improved sagittal balance. eZspand™ does not require secondary locking, minimizing procedural steps. Each implant expands to 4.5mm from … [Read more...] about eZspand™
EXCENDER
The EXCENDER is an interbody fusion cage made from a titanium alloy that complies with ASTM F136 standards. This product is specifically designed for use in Transforaminal Lumbar Interbody Fusion (TLIF) procedures to address spinal disorders, particularly in patients with damaged discs. The EXCENDER is intended for implantation in the lumbar region to facilitate spinal … [Read more...] about EXCENDER
EquiLOX Expandable Lumbar Cage
EquiLOX Expandable Lumbar Cage Technology incorporates an innovative design, maximizing expansion without sacrificing through-cage fusion area, with a highly efficient workflow. *Custom options available upon request**7mm cage expands 4mm to max height of 11mm About Captiva Spine Captiva Spine, founded in 2007, is a privately held medical device company that brings … [Read more...] about EquiLOX Expandable Lumbar Cage
eFuse® expandable
eFuse® expandable interbody cages with surface technology provides a minimally invasive solution for TLIF Oblique and PLIF as well as for endoscopy procedures with in situ expansion for restoration of normal anatomic disc height and decompression of neural structures.Streamlined instrumentation achieves minimal tissue disruption and nerv retraction while acieving surgical goal … [Read more...] about eFuse® expandable
dualX™ Dual Expanding Interbody Fusion System
The dualX technology is comprised of a family of titanium expandable interbody devices designed to be implanted in lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) spinal procedures. The dualX portfolio contains varying footprints, heights and degrees of lordosis with post-expansion bone grafting … [Read more...] about dualX™ Dual Expanding Interbody Fusion System
Explorer® TO Expandable Interbody System
Explorer® TO Expandable Interbody was designed to minimize impaction forces required with traditional static implants. The implant seamlessly expands within the disc space to restore height and lordosis optimized for each individual patient. The system consistently deliver autograft and/or allograft to fill any voids left in the implant from device expansion. With both a height … [Read more...] about Explorer® TO Expandable Interbody System
FlareHawk9™
FlareHawk9™ is a multidirectional expandable lumbar fusion device, featuring our innovative Adaptive Geometry™, that can be inserted at a low profile of 7mm or 9mm tall by 9mm wide before expanding up to 14mm tall and 14mm wide. Features: About Expandable Cages Expandable interbody cages allow surgeons to go through a small corridor and change the geometry of … [Read more...] about FlareHawk9™
FlareHawk7™
FlareHawk7™ is a multidirectional expandable lumbar fusion device, featuring Accelus’s innovative Adaptive Geometry™, that can be inserted at a low profile of 7mm tall by 7mm wide before expanding up to 12mm tall and 11mm wide. Its minimal insertion profile and instrumentation is design to respect the patient’s anatomy while facilitating the surgeon’s preferred … [Read more...] about FlareHawk7™
FORZA XP Expandable Spacer System
The FORZA® XP Expandable Spacer System offers titanium alloy expandable interbodies for Posterior Lumbar Interbody Fusion (PLIF) and Transforaminal Lumbar Interbody Fusion (TLIF) procedures. After insertion into the disc space, the interbodies can be expanded to fit the patient anatomy. Unlike many competitive expandables that trade off graft space in order to stay within the … [Read more...] about FORZA XP Expandable Spacer System
ELSA®
ELSA® is an expandable interbody fusion spacer with integrated fixation designed to maximize segmental lordosis while minimizing disruption to patient anatomy. ELSA VIDEO ANIMATION MARS™3V VIDEO ANIMATION MARS™ 3VL VIDEO ANIMATION Benefits: Adjustable Lordosis: Implants with adjustability from 5° to 20° or 15° to 30° allow for insertion at a smaller starting height … [Read more...] about ELSA®
FLXfit15™
The FLXfit™ is an expandable, articulated interbody fusion device (IBFD) used in conjunction with supplemental fixation to provide structural stability in skeletally mature individuals following total or partial discectomy.The FLXfit™ is a unique titanium cage which articulates laterally as well as expands in height. This enables larger footprint support as well as … [Read more...] about FLXfit15™
ELSA®-ATP
ELSA®-ATP is an expandable lateral system designed to provide access to the lumbar spine anterior to the psoas muscle. Entering the disc space from this approach helps to avoid complications associated with the lateral trans-psoas approach and the lumbar plexus. This anterolateral working corridor also allows for direct access to L4‑L5 where a high iliac crest may complicate … [Read more...] about ELSA®-ATP
MAGNIFY®-S
MAGNIFY®-S is an all titanium expandable ALIF device designed to maximize indirect decompression, restore disc height, and optimize fusion potential. Minimized insertion height and continuous expansion combined with the ability to pack additional bone graft after expansion makes MAGNIFY®-S an ideal choice for stand-alone ALIFs. Benefits: Maximized Indirect … [Read more...] about MAGNIFY®-S
MAGNIFY®
The MAGNIFY® expandable ALIF spacer system is designed to minimize impaction, maximize indirect decompression, and optimize fusion. Minimized insertion height, continuous expansion, and the ability to backfill autogenous bone graft separates MAGNIFY® from from traditional ALIF spacers. Benefits: Minimize Impaction: The reduced insertion height eases … [Read more...] about MAGNIFY®
ORIGAMI Expandable Cage
Origami is a platform of Disruptive Designs for Expandable Interbody Cages. Origami VIDEO ANIMATIONOrigami-Lateral-Cages-Brochure.Twist Technologies.pdf Features: A Platform for open surgery or minimal accessfor TLIF, Lateral, ALIF & PLIF approachesdiverse choices of materials A Variety of Cage Designs 1 or 2 extensionsa choice of various … [Read more...] about ORIGAMI Expandable Cage
PROLIFT® Lateral
The PROLIFT e-LIF Spacer System with Osseo-Loc Surface Technology offers a Micro Invasive solution for Lateral Lumbar Interbody Fusions. With up to 6mm of in situ expansion and up to 15˚ of lordosis, ProLift allows for restoration of patient disc height and decompression of neural elements. With the small starting height options and maximum end plate coverage, it is … [Read more...] about PROLIFT® Lateral