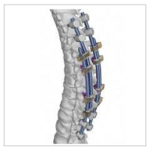
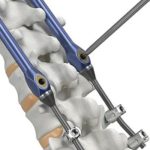
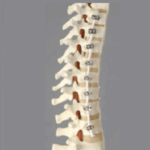
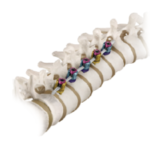
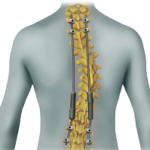
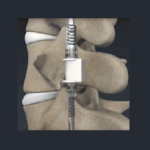
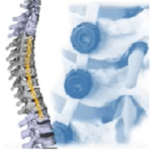
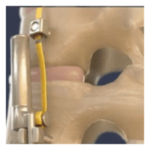
VerteGlide™ is a unique guided growth treatment option for skeletally immature patients with progressive scoliosis. This intuitive technique involves a sliding screw construct to allow for patients to grow at their own natural pace. This intuitive technique involves a sliding screw construct to allow for patients to grow at their own natural pace. VerteGlide is a guided … [Read more...] about VerteGlide™
Early Onset Scoliosis (EOS)
Luque Trolley
The Modern Luque Trolley technique was implemented in 2011, updating the technique invented by Eduardo Luque forty years back. The Modern Luque Trolley is performed by fixing one pair of rods proximally and the other pair of rods distally with the help of apical gliding anchors. The proximal and distal rods grow with the spine while the apical gliding anchored still. About … [Read more...] about Luque Trolley
MARVEL™ Growing Rod System
The MARVEL™ Growing Rod System is designed for patients under the age of 10 with early onset scoliosis to obtain and maintain correction while allowing continued growth through minimally invasive distraction. The expansion mechanism provides a large distraction range and tactile feedback during expansion. Features and Benefits About Globus Medical Globus Medical, … [Read more...] about MARVEL™ Growing Rod System
The Tether™ – Vertebral Body Tethering System
Zimmer Biomet’s anterior vertebral body tethering (AVBT) solution, The Tether, uses a strong, flexible cord made of SULENE® PET (polyethylene-terephthalate) , rather than metal rods, to pull on the outside of a scoliosis curve to initially straighten the spine, while the inside of the curve is left free to grow. This growth modulation approach now offers select, well-indicated … [Read more...] about The Tether™ – Vertebral Body Tethering System
REFLECT™
REFLECT™ is an anterior scoliosis correction system that is used to apply fixation on the convexity of the scoliotic vertebrae with a flexible cord to limit growth on the convex side and allow unilateral growth on the concave side.Device is not cleared by the FDA yet Advantages: About Globus Medical Globus Medical, Inc. is a leading musculoskeletal … [Read more...] about REFLECT™
MAGEC®
NuVasive Inc. purchased Ellipse Technologies Inc. in 2016. Ellipse was the developer and marketer of a novel platform of magnetically adjustable implant systems based on its MAGnetic External Control, or MAGEC®, technology. MAGEC technology implants are adjustable at the time of implantation and are distracted non−invasively over the course of treatment to … [Read more...] about MAGEC®
FLYTE
Comprising a team of elite engineers and orthopedic industry experts, AMB Surgical is devoted to the development and commercialization of its FLYTE™ smart automated growing rod technology which holds promise to reduce anxiety and trauma from juvenile and adolescent surgeries. Device is not cleared by the FDA yet FLYTE is the next generation growing rod … [Read more...] about FLYTE
MIScoli™
The MIScoli™ system is one of a few VBT devices that are under investigation, or limited market release, in many countries. Various medical device developers have committed to developing VBT solutions to serve an unmet need in the scoliosis surgical treatment market. The MIScoli System was honored to have been designated as a Breakthrough Device by the US Food & Drug … [Read more...] about MIScoli™
Auctus Dynamic Tethering System
Auctus Surgical, a company devoted to non-fusion options for scoliosis patients, is developing a one-of-a-kind vertebral body tethering (VBT) system to treat pediatric scoliosis with a non-fusion, dynamic approach. The company is announcing the recent Breakthrough Device Designation by the FDA, a recognition that the Auctus dynamic vertebral body tethering system is unique … [Read more...] about Auctus Dynamic Tethering System
Spring Distraction System
Cresco’s revolutionary Spring Distraction System™ for early onset scoliosis grows with the spine while correcting the various planes through continuous distraction. This allows for fewer interventions and associated collateral damage, resulting in a better outcome and quality of life.The Spring Distraction System™ (SDS™) is part of this family of systems. It allows spinal … [Read more...] about Spring Distraction System
CurvRITE
CurvRite is the Next Generation Technology for Scoliosis Patients based on 3D Growth Guidance Rod System: CurvRITE = Curve + Right We correct the CURVed spine to physiological, nature curvature which is the RIGHT way by using our proprietary 3D correction technology, while traditional 2D distraction corrects the CURVed spine in a compromised way which causes Flat back syndrome, … [Read more...] about CurvRITE
KYRA® Correction Cord System
KYRA® Correction Cord System is a fixing system which includes various types and sizes of pedicle screws, cord and nut.KYRA® Correction Cord System is the medical device which treats scoliosis while a person is actively growing and uses their own growths to repair their curves.KYRA® Correction Cord System is a spine device which has been designed for the treatment of idiopathic … [Read more...] about KYRA® Correction Cord System
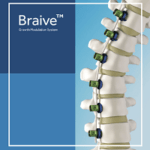
Braive
In September, 2021 Medtronic announced that it has enrolled its first patient and completed the first surgical procedure in its BRAIVE™ IDE study, which will evaluate the safety and effectiveness of the Braive™ growth modulation system for treatment of progressive juvenile (JIS) or adolescent (AIS) idiopathic scoliosis. The first patient was recruited by The Newcastle Upon Tyne … [Read more...] about Braive
NEMOST
The NEMOST implant is a sterile, single-use device, intended to be surgically implanted via a posterior approach.It is a growth implant intended for pediatric use in children with RISSER 0 and as first intention surgery.The desired effect is a regular lengthening of the device to obtain a gradual correction of the spinal deformity over time while allowing the continued growth … [Read more...] about NEMOST
SHILLA ™ Growth Guidance System
Early onset scoliosis (EOS) is a complex spinal deformity that which remains an unsolved problem in the early growing years represents. Frequent observations and follow-up checks with suitable Measurement methods are important in order to obtain the best possible treatment Correction and stabilization of the spine until the final Ensure skeletal maturity. If the EOS is not … [Read more...] about SHILLA ™ Growth Guidance System
VEPTR
VEPTR is based on a three-dimensional thoracic approach to treat patients with complex chest wall and/or spinal deformities where the thorax is unable to support normal respiration or lung growth (Thoracic Insufficiency Syndrome). Additionally VEPTR devices control and may correct scoliosis. VEPTR is designed to mechanically stabilize and distract the thorax to improve … [Read more...] about VEPTR