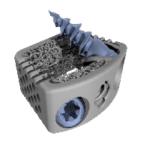
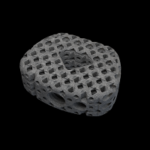
Utilizing high-definition 3D printing, HiveTM Interbody Fusion Devices all feature a Soft Titanium® lattice core with HD’s 4 evolutionary technologies. The HiveTM family includes multiple footprint and lordosis options for each surgical approach. Hive Cervical-Brochure.HD LifeSciences.pdf Benefits: Reduced Stiffness: HiveTM interbodies using Soft Titanium® technology … [Read more...] about HiveTM C
3D ACIF
HEDRON IC™
HEDRON IC™ is a 3D printed ACDF spacer that can be used as an interbody spacer itself or combined with the COALITION AGX Plate and screws as a stand-alone plate-spacer. HEDRON IC™ features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant. Features: The Face of Fusion:An ovine interbody study demonstrated significantly more … [Read more...] about HEDRON IC™
F3D C2 Stand Alone Cervical
The F3D-C2 Stand-alone Cervical System is an interbody fusion system comprised of a spacer with two integrated bone screws secured by a locking mechanism within the cage. F3D C2 Stand ALONE Cervical VIDEO ANIMATION Benefits: Streamlined construct features biomechanical benefits of Mimetic Metal® 3D printing technologyScrews anchor through zero-step anti-back-out … [Read more...] about F3D C2 Stand Alone Cervical
GENOSS 3d Cage™ Cervical Cage
A cage-like agglutinating prosthesis used to treat structural anomalies caused by degenerative disc herniation of the cervical spine during an orthopedic or neurosurgery procedure, GENOSS 3d Cage™ Cervical Cage is a spinal cage that is transplanted between bones or artificial bone implants in order to provide sufficient space for mechanic safety or interspinous agglutination. … [Read more...] about GENOSS 3d Cage™ Cervical Cage
IdentiTi™ C
ATEC’s IdentiTi Cervical Porous Ti Interbody Implants are designed to provide the biological, biomechanical, and imaging characteristics that surgeons seek in a fusion construct. The subtractive process used to manufacture each IdentiTi Implant results in more predictable mechanical performance and enhanced imaging characteristics. IdentiTi implants take advantage of bone’s … [Read more...] about IdentiTi™ C
Monza
Monza is an innovative cervical cage that, thanks to its trabecular titanium structure made with the most modern 3d printing techniques, guarantees immediate and safe mechanical stability and certain osseointegration to all types of implants. The cervical cage was developed to cover all possible sizes and degrees of lordosis and allows … [Read more...] about Monza
Modulus C
Unlike any other 3D printed titanium implant on the market, Modulus is designed through a proprietary optimization algorithm that balances strength and radiolucency. MODULUS C VIDEO ANIMATION Advanced Materials Science™Adhering to the three core principles of Advanced Materials Science—Surface, Structure, and Imaging—NuVasive has pioneered design and manufacturing methods … [Read more...] about Modulus C
Mecta-C Stand Alone Interbody Fusion
The Medacta Mecta-C Stand Alone Interbody Fusion Device offers an a MODULAR design that incorporates the benefits of an anterior plate and a radiolucent interbody spacer. In order to accommodate specific anatomical requirements and specific pathologies to treat, the surgeon has the ability to assemble any of the available plates intraoperatively giving the … [Read more...] about Mecta-C Stand Alone Interbody Fusion
Monet™ Anterior Cervical Fusion system
The Monet™ Anterior Cervical Fusion system is designed for intra-operatively flexibility. The cage component can be implanted independently or in conjunction with the Monet™ supplemental fixation plates, making it a truly comprehensive Anterior Cervical Fusion solution. Features: CTL Amedica CTL Amedica is a forward thinking medical device design, development, and … [Read more...] about Monet™ Anterior Cervical Fusion system
neoWave™ C
neoWave™C is a 3D-printed interbody fusion device featuring its repeating waveform matrix technology that is designed to support postoperative osseointegration while uniquely diffusing compressive loads to reduce device stiffness and, therefore, the risk of subsidence. The proprietary waveform technology also functions to disperse loads during device malleting to meet the … [Read more...] about neoWave™ C
NEXXT MATRIXX® Stand Alone Cervical System
Designed to be used as an adjunct to fusion at one or two contiguous levels (C2-T1) in skeletally mature patients, the Stand-Alone Cervical System will help patients that suffer from the treatment of degenerative disc disease. It should be used with the bone screw fixation provided and requires no additional fixation. The increasing adoption of additive manufacturing into … [Read more...] about NEXXT MATRIXX® Stand Alone Cervical System
OYSTER ACIF
State-of-the-art production technology ,3D titanium printing enables an open-pore cage design with a mesh macrostructure composed of approximatively 73% air and 27% titanium. Good primary stability thanks to teeth and spikesAvailable in anatomical as well as wedge-shaped designEasy and intuitive instrumentation and disassembly of instruments Benefits: Implant Porosity … [Read more...] about OYSTER ACIF
Polar-b Cervical Bladed Peek Cage
Polar-b-SGT.norm.pdf Featutes: Locking blade mechanismTantalum markerThreaded surface2 angle for compatibility with anatomyProvide saving of time and facility with applications methodLarge graft area About norm NORM Medical Devices Co. Ltd. was founded in 2006 in Ankara, the capital city of Republic of Turkey with a branch Office Istanbul, by its current General … [Read more...] about Polar-b Cervical Bladed Peek Cage
SPIRA- C Integrated Interbody system
The SPIRA ® -C Integrated Fixation System consists of a stand-alone interbody fusion device with internal screw fixation,for one or two levels from the C2-C3 disc to the C7-T1 disc. Features: About Camber Spine Camber Spine Technologies LLC is a medical device company focused on the design, development and commercialization of innovative and proprietary … [Read more...] about SPIRA- C Integrated Interbody system
SureMAX Cervical Spacer
SureMAX is a 3D printed, titanium alloy Cervical Spacer System FDA cleared. The 3D printed, titanium alloy SureMAX Cervical Spacer offers a roughened porous surface with several unique features on the superior, inferior, and lateral aspects of the implant, designed to effectively engage bone on the vertebral endplates. These features combine to provide an ideal environment for … [Read more...] about SureMAX Cervical Spacer
SPIRA C Integrated
Camber Spine, announced last week the FDA clearance and nationwide launch for The SPIRA®– C Integrated Interbody system, a stand-alone integrated fixation system, SPIRA C VIDEO ANIMATIONSpira-C-Brochure.Camber Spine.pdf The SPIRA®-C Integrated incorporates Camber Spine’s proprietary technology Surface by Design®, a proven osteopromotive surface, according to a recent … [Read more...] about SPIRA C Integrated
Shoreline RT®
The Shoreline RT® anterior cervical interbody system was designed for surgeons to have ultimate system flexibility, construct modularity, and refined instrumentation. Utilizing NanoMetalene® Technology, Reef Topography™, and an optional TruProfile® plating system, Shoreline RT provides complete intraoperative choices. Features: About … [Read more...] about Shoreline RT®
TrellOss™-C
TrellOss-C is a 3D printed titanium interbody platform featuring a scaffold structure with 70% porosity and a 7 micron roughened surface topography to foster a cellular relevant environment for adhesion and bone ingrowth. TrellOss-C-Brochure.Zimmer-Biomet.pdfTrellOss-C-SGT.Zimmer-Biomet.pdf Features: Rigid teeth help to resist implant migrationCentral window for graft … [Read more...] about TrellOss™-C
Tritanium C
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing. Tritanium-C-Brochure.Stryker.pdf This cage is available in a variety of footprints, heights and lordotic angles to accommodate various patient anatomies. It features a large, … [Read more...] about Tritanium C