Lumbar fusion has been—and continues to be—the standard treatment for degenerative lumbar spine conditions associated with instability and pain. However, one of its unintended consequences is the loss of motion at the treated segment, which may accelerate adjacent segment degeneration and limit patient function. To avoid these drawbacks and offer an alternative capable of preserving mobility without sacrificing stability, Total Facet Arthroplasty Systems (TFAS) were developed.
These systems were designed to replace the facet joints after wide decompression, with the goal of restoring articular function, maintaining range of motion, and providing segmental stability without the need for fusion. In vitro studies on human lumbar spines showed that TFAS devices could reestablish a motion pattern close to physiological behavior in flexion, extension, lateral bending, and axial rotation—unlike fused segments.
Despite their biomechanical promise, TFAS encountered significant limitations. Available clinical evidence is scarce and largely based on small case series or isolated reports, and several clinical trials were discontinued. Mechanical complications, such as component fractures, were also documented. No system obtained FDA approval for commercial clinical use. Some devices progressed through regulatory pathways outside the United States, but none achieved broad commercialization.
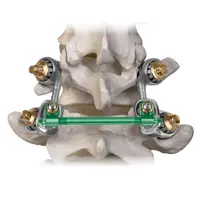
One example of this technological evolution was the Acadia Facet Replacement System (Globus Medical), a later development aimed at more accurately reproducing the anatomy of the lumbar facets. Like TFAS, Acadia sought to restore joint function and segmental motion after decompression. Although early biomechanical studies demonstrated improvements in kinematics, clinical adoption remained limited, and no large-scale trials support its use. It also failed to achieve widespread regulatory approval or commercial presence. Nevertheless, Acadia contributed valuable insights that influenced subsequent dynamic stabilization and joint replacement technologies.
Evolution Toward Current Systems: TOPS and MOTUS
The accumulated experience with TFAS and Acadia paved the way for more advanced devices:
TOPS System (Total Posterior Spine System)

TOPS is an FDA‑approved posterior dynamic stabilization system. Its design enables controlled segmental motion and stabilizes the posterior elements after decompression without requiring rigid fusion. The device mechanically guides flexion, extension, lateral bending, and axial rotation through a posterior articulating mechanism. Importantly, TOPS is not an anatomic total joint replacement: it does not replace the disc or the facets. Instead, it preserves functional mobility while maintaining segmental stability. Mid‑term clinical studies support its effectiveness in reducing pain and maintaining motion.
MOTUS Total Joint Replacement (3Spine)

The MOTUS system represents a different conceptual approach: it is the first lumbar total joint replacement designed to replace the functional spinal unit (FSU) as a motion‑preserving alternative to fusion for degenerative lumbar spondylolisthesis and stenosis. Unlike dynamic stabilization devices, MOTUS replaces both the disc and the facet joints with a single articulating implant that restores controlled motion in all planes.
Key characteristics include:
- True total joint replacement of the lumbar motion segment
- Restoration of physiological kinematics, including coupled motion
- Load sharing across the implant to mimic natural biomechanics
- Stability without rigid fixation, aiming to reduce adjacent segment degeneration
- Designed for single‑level L4–L5 disease, the most commonly affected segment
MOTUS has undergone clinical evaluation through the FDA’s Breakthrough Device Program. Early clinical results suggest improvements in pain, function, and mobility compared with fusion, although long‑term data are still being collected. If ongoing trials confirm its safety and durability, MOTUS may become the first widely adopted lumbar total joint replacement system.
###
