ATLANTA, June 11, 2020 /PRNewswire/ — MiRus is pleased to announce that it received FDA 510(k) clearance for its 3DR™ (Randomized) Printed Lumbar Interbody Fusion System which consists of the CALLISTO 3DR™ PLIF, HYPERION 3DR™ TLIF, CALYPSO 3DR™ LLIF and the ANTARES 3DR™ ALIF. “The HYPERION 3DR TLIF is the first interbody with stiffness equivalent to […]
NEWS
OrthoPediatrics Corp. Commences Initial U.S. Launch of ApiFix’s FDA-Approved Spinal Deformity Correction System
WARSAW, Ind., June 10, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq:KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, is pleased to announce the initial U.S. launch of the ApiFix Minimally Invasive Deformity Correction (“ApiFix”) system. Additionally, the Company anticipates approximately 20 leading clinical centers in the United States to […]
RTI Surgical Holdings, Inc.® Bolsters Spine Business with Key Senior Leadership Appointments
DEERFIELD, Ill., June 10, 2020 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, today announced two senior leadership appointments to support its transition to a pure-play global spine company. Scott Durall will join RTI as Chief Commercial Officer, and Bryan Cornwall will join RTI as Executive Vice President, Research […]
4WEB Medical Announces FDA 510(k) Clearance of Stand-Alone Anterior Lumbar Interbody Fusion Device
DALLAS, June 9, 2020 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device (ASTS-SA). The new […]
Dymicron™ Expands Leadership Team, Adds Significant Experience of Industry Veterans Ted Bird and Eric Lange
With 50+ years of combined spine industry and clinical experience, Bird and Lange will drive development and commercialization of the company’s next-generation cervical disc replacement system OREM, Utah, June 9, 2020 /PRNewswire/ — Dymicron, a privately held medical device company developing the next-generation Triadyme-C™ artificial cervical disc, has strengthened its leadership team with the appointments of Ted Bird as Executive […]
Spineart, the fast-growing spinal devices company, welcomes EGS Beteiligungen AG which invests CHF 50 million of growth equity
Spineart today announces that it secured a CHF 50 million investment from EGS, which thus becomes a cornerstone investor in the company next to the Management and Gimv, its largest shareholder. These proceeds will be used to implement strategic clinical studies, accelerate Spineart’s organic growth in key markets, seize selective acquisition opportunities and for continued […]
SpineGuard Files Its “510K” Dossier for the Clearance of Its New “DSG Connect” Platform in the United States
SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the filing of its “510K” regulatory dossier with the FDA, seeking the authorization to commercialize its DSG Connect platform in the USA. Already being utilized experimentally in a […]
Meditech Spine Receives FDA Clearance for its CURE™ OPEL-L (S) system
ATLANTA, June 1, 2020 /PRNewswire/ — Meditech Spine has received FDA 510(k) clearance to market the CURE™ Opel-L (S) system, a new lumbar plate option which expands upon the previously cleared CURE™ LP Plate System and compliments its Talos®-A (HA) Interbody system. With this approval, Meditech will now offer an Interbody/Plate assembly for the anterior lumbar spine. By […]
FDA Grants Clearance on Nexxt Spine ALIF and Lateral Systems
May 28, 2020- Noblesville, IN– Nexxt Spine LLC, a leading manufacturer of spinal surgery solutions is proud to announce the U.S. Food and Drug Administration (FDA)510(k) clearance of two lumbar based systems; ALIF and Lateral. This milestone will allow the company to commence in-house manufacturing of the two systems and plan for subsequent alpha launches […]
Life Spine Announces Initial Cases With the DYNA-LINK® Ti Stand-Alone ALIF Spacer System
Huntley, IL, May 28, 2020 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today the first surgeries with the DYNA-LINK Ti Stand-Alone ALIF Spacer System. “The DYNA-LINK Ti System instrumentation and implants provided excellent intra and post-operative results for my patient requiring […]
Centinel Spine Announces FDA Approval for the Manufacturing Transfer of prodisc® Technology
New York, NY, May 27, 2020 – Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced FDA approval for the manufacturing transfer of both the prodisc® C Cervical Total Disc Replacement and prodisc® L Lumbar Total Disc Replacement systems to new strategic vendors. The FDA approval for manufacturing transfer is a critical […]
RTI Surgical Holdings, Inc.® Appoints Stuart F. Simpson to Board of Directors
DEERFIELD, Ill., May 26, 2020 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, today announced the appointment of Stuart F. Simpson as an independent member of its board of directors. Mr. Simpson most recently served as the President of the Joint Replacement Division of Stryker Corporation (NYSE: SYK), a […]
Zavation Medical Products, LLC. Launches eZspand™ Expandable Cage
JACKSON, Miss., May 26, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of eZspand™, an expandable lumbar interbody fusion device. eZspand™, the latest addition to Zavation’s portfolio, features unmatched expandable precision paired with continual expansion […]
4 British Spinal Companies to Know…!
According to Mordor Intelligence, the UK spinal surgery devices market will show a rapid growth due to increasing incidences of degenerative spinal conditions and technological advances in spinal surgery. Owing to the recent technological advances, the number of minimally invasive procedures has increased significantly, along with the traditional open surgery techniques.In the United Kingdom, spinal pain […]
Nanovis Awarded 510(k) for Nano FortiFix® the First Nanotechnology Enhanced Pedicle Screw System.
CARMEL, Indiana (April 29, 2020) – Nanovis today announced that it received 510(k) clearance for a bioceramic nanotube surface on its Nano FortiFix Pedicle Screw System. “Nanovis continues to advance innovative technology platforms to improve patient care. We are very pleased to receive the first FDA clearance for a nanotechnology enhanced pedicle screw system. This technology […]
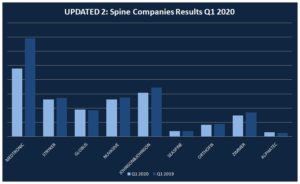
Updated II: How much the main Spine Companies have lost because of COVID-19 in Q1 2020?
The COVID-19 has been caused measurable impact on the global economy and, in turn, on the Spinal Fusion market. Quarantines, traveling constraints, and social distancing measures on a broad-scale drive a steep decline in business and consumer spending until the end of Q2. All the companies have taken mesures to protect the healths of their […]
Medtronic Reports Fourth Quarter and Fiscal Year 2020 Financial Results; Announces Dividend Increase
DUBLIN, May 21, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced financial results for its fourth quarter and fiscal year 2020, which ended April 24, 2020. The results were in-line with the update the company provided on April 21, 2020, which detailed the impact of the COVID-19 pandemic on its operations and financials. Medtronic’s results were also […]
New Directional Cannula Provides More Flexibility and Control to Balloon Kyphoplasty Procedures
DUBLIN, May 19, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. launch of Kyphon Assist™ Directional Cannula for use with its balloon kyphoplasty (BKP) products to treat vertebral compression fractures due to osteoporosis, cancer or benign lesions. This innovative new device allows physicians greater control when inflating the bone tamp while […]
NuVasive LessRay® Platform Wins Best New Imaging Technology Solution in 2020 MedTech Breakthrough Awards
SAN DIEGO, May 19, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced its LessRay® radiation reduction and workflow enhancement platform won the “Best New Imaging Technology Solution” award in the 2020 MedTech Breakthrough Awards. The MedTech Breakthrough Awards recognize innovative companies, […]
Cerapedics Announces Results from Clinical Trial of i-FACTOR® Peptide Enhanced Bone Graft in Lumbar Fusion Surgery
WESTMINSTER, Colo., May 19, 2020 /PRNewswire/ — Cerapedics, a private ortho-biologics company, today announced that results from a clinical trial evaluating i-FACTOR Peptide Enhanced Bone Graft in non-instrumented lumbar fusion surgery has been published in the May 2020, Volume 20, Issue 5 print of The Spine Journal as the lead article. The data demonstrate that elderly patients in Denmark treated with i-FACTOR bone […]
6 Stand Alone ACIF 3D Printed Titanium Cages to know…!
The global Cervical Interbody Fusion Cages market is growing. According to reportsweb.com, it will reach 1120 million US$ by the end of 2025, growing at a CAGR of 4.0% during 2018-2025.In recent years, the trend in the cervical spine market has been the use of stand-alone cages as it offers numerous advantages. On the other hand, […]
Swiss Medtech Neo Medical Raises CHF 13.2M for its Disruptive Controlled-Fixation Solutions for Spinal Fusion Surgery
The company welcomes two prominent Medtech figures to its Board of Directors Neo Medical closes a CHF 13.2M financing round to support the growth of its Controlled-Fixation solutions for spinal fusion surgery Neo Medical welcomes Michel Orsinger and Oern Stuge,two highly recognized worldwide industry veterans, to its Board of Directors LAUSANNE, 15May 2019: Neo Medical, […]
ATEC Reports First Quarter 2020 Financial Results And Recent Corporate Highlights
CARLSBAD, Calif., May 11, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a provider of innovative spine surgery solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended March 31, 2020, and recent corporate highlights. First Quarter 2020 Financial Results Total revenue of $30.1 million; U.S. revenue of $29.1 million, up 27% […]
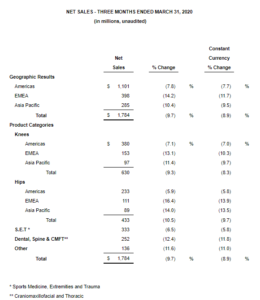
Zimmer Biomet Announces First Quarter 2020 Financial Results
WARSAW, Ind., May 11, 2020 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today reported financial results for the quarter ended March 31, 2020. The Company reported first quarter net sales of $1.784 billion, a decrease of 9.7% from the prior year period, and a decrease of 8.9% on a constant currency basis – both consistent with the […]
Camber Spine Technologies Announces Nationwide Launch of SPIRA®-C Integrated and FORTICO™ Anterior Cervical Plate
KING OF PRUSSIA, Pa., May 7, 2020 /PRNewswire/ — Camber Spine, a leading innovator in spine and medical technologies, today announced the FDA clearance and nationwide launch for two novel anterior cervical products: The SPIRA®– C Integrated Interbody system, a stand-alone integrated fixation system, and the FORTICO™ Anterior Cervical Plating System, a two screw plating system intended for […]
Globus Medical Reports First Quarter 2020 Results
AUDUBON, Pa., May 07, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the first quarter ended March 31, 2020. Worldwide sales were $190.6 million, an increase of 4.2% as reported First quarter net income was $25.9 million Diluted earnings per share (EPS) was $0.25 and non-GAAP […]
Xtant Medical Announces First Quarter 2020 Financial Results
BELGRADE, Mont., May 07, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the first quarter ended March 31, 2020. First Quarter 2020 Financial Highlights: Revenue for the first quarter of […]