In 2021, we have seen fewer acquisitions than in previous years. It seems that companies in the COVID era have thought twice about making them. However, we are sure that 2022 will be a busy year in this regard. Do not forget that although the spine market continues to grow, acquiring competitors is the fastest way to grow and increase market share. Just as an example, the … [Read more...] about What have been the most relevant acquisitions in 2021 in the spine market?
NEWS
New products in 2021? Learn about more than 40 new devices and 35 releases …!
In 2021, at least 44 spine products were launched on the market. According to our information, the companies that have been most active in 2021, launching more than two new products have been: Tsunami Medical, Seaspine, Spine Wave, Clariance Spine, Camber Spine, Medtronic, ATEC, Intelivation and, Nuvasive. These companies represent 66% of all launches this year (Chart … [Read more...] about New products in 2021? Learn about more than 40 new devices and 35 releases …!
Globus in takeover talks with NuVasive to boost spine portfolio
The J.P.Morgan analysts see "significant challenges" to the potential acquisition of NuVasive because of the nature of the spine market. Globus will need to weigh whether the potential dis-synergies offset the chance to cut costs. "It's worth reiterating that 'one + one' rarely gets to 'two' on the top line, with significant portfolio overlap often coupled … [Read more...] about Globus in takeover talks with NuVasive to boost spine portfolio
Are you thinking of changing jobs? Do you want to hire someone for a position in spine? We have the updated list of 50 Headhunters that could help you! (Updated!)
In these tough times, you may need to look for a new job or you may be looking to strengthen your team. We believe that we can help you with something. We have updated the list of headhunters that we had published. In it, you can find 50 recruitment companies. Most of them are centered on medical devices among other sectors. Others are very focused on orthopedics or even the … [Read more...] about Are you thinking of changing jobs? Do you want to hire someone for a position in spine? We have the updated list of 50 Headhunters that could help you! (Updated!)
The German Spine Market. What is the sales volume? Which are the German competitors?
The Germany spinal surgery market is moderately competitive and consists of several major players that hold substantial shares as Depuy Synthes, Zimmer Biomet, Stryker, Medtronic, Aesculap and Globus Medical. Moreover, with the increasing technological advancements and product innovations, mid-size to smaller companies are increasing their market presence by introducing new … [Read more...] about The German Spine Market. What is the sales volume? Which are the German competitors?
Which is our Spine Market Share Estimates for the End of 2021? How has the First half of the year been for the Key Companies?
2021 in the Spine market is being a year of growth after the impact generated by COVID in 2020. Today we are going to analyze (with the Q1 and Q2 already published), the changes in market shares and which companies are recovering faster after the pandemic. As you will see, we have included all the data for the first semester of 2021 compared to those of 2020 for the main … [Read more...] about Which is our Spine Market Share Estimates for the End of 2021? How has the First half of the year been for the Key Companies?
I want a robot for spinal surgery! What are the best options?
The growing awareness among the physicians and patients regarding the benefits associated with the usage of spine surgery robots has been directly impacting the growth of the market. As we have already commented in another article, Spine surgery robots market account to USD 330.85 million by 2027 growing at a CAGR of 16.0% 2020-2027. It seems clear that robotics is the future … [Read more...] about I want a robot for spinal surgery! What are the best options?
UPDATED: Do you urgently need a surgical technique? Try here! We already have more than 725!
Today we have UPDATED the new section called BROCHURES where you can find 726 brochures and surgical techniques for the different products on the market. Now, everything is located in the same place.We believe that it is a very useful information when you want to find information about a product that is no longer on the market or simply when you urgently need an specific … [Read more...] about UPDATED: Do you urgently need a surgical technique? Try here! We already have more than 725!
Which is the Spine Market First Estimate for 2021?
In this article, we want to present our growth forecasts for the main spine companies in 2021. Please, bear in mind that it is a first estimate made by us and that it may not be fulfilled. Companies remain subject to the potential and uncertain impact of the ongoing COVID-19 pandemic. Hospitals may experience a surge in COVID-19 cases and defer elective procedures so that … [Read more...] about Which is the Spine Market First Estimate for 2021?
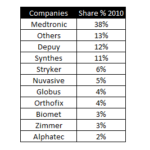
How Market Shares and competitor positions have changed in Spine in the last Ten years?
A few days ago we published an article about the market shares of the spine in 2020. Today, we received a comment suggesting us (Thanks to Jon Wait from Aurora Spine!) to compare them with the situation of 10 years ago. Fortunately, we have that information, and we have done it. The results are the following: Medtronic: It continues leading the market, but it has lost 10 … [Read more...] about How Market Shares and competitor positions have changed in Spine in the last Ten years?
How have the market shares in the spine market changed after 2020 with COVID-19?
These days, most companies are publishing their 2020 results. In this article, we have collected all of them to measure the impact of Covid on all of them. It has not been easy as each company measures and presents the spine results differently. According to Research and Markets, the global market for the Spine in 2019 was USD 9662 million. In 2020, due to COVID.19, it has … [Read more...] about How have the market shares in the spine market changed after 2020 with COVID-19?
Why have Zimmer Spun off its Spine Business? What could happen now?
Last week Zimmer Biomet announced its intention to spin off its Spine and dental businesses. According to Bryan Hanson, CEO of the company, the goal is to focus on high-growth and priority areas such as knees, hips, S.E.T. and CMFT, prioritize resources and simplify their operating models. From our point of view, this strategic action of focusing on the markets … [Read more...] about Why have Zimmer Spun off its Spine Business? What could happen now?
Quiet People in Meetings Are Incredible
Tim Denning (medium.com)--Knowing when not to talk is an art. As a corporate man by day, and an entrepreneur by night, I’ve attended my fair share of meetings over the last decade or so. Meetings can be an odd experience. Before you know it the meeting can get out of control. Leaders with pinstripe suits or hair that is turning grey quicker by the day can lose the plot. … [Read more...] about Quiet People in Meetings Are Incredible
Someone has to say it! Thank you Mr Demski!
A few days ago, I found this letter from the CEO of Globus Medical on Linkedin. It seemed to me that finally someone important had something to say and said it. We do not work at Globus Medical, nor for them, but Dave Demski undoubtedly has our respect and admiration. The letter was the following: Dear Globus Team, The tragic and inexcusable death of George Floyd in … [Read more...] about Someone has to say it! Thank you Mr Demski!
5 More Company Logos that you will probably never see again…
Many acquisitions have taken place in the Spinal market in the last 10 years. Those companies acquired, have disappeared forever but today as we did in our article "5 Company Logos that you will probably never see again…" , we want to remember another Five Companies: 1.- Lanx Lanx was founded by a team of experienced medical device professionals and engineers in 2003 to … [Read more...] about 5 More Company Logos that you will probably never see again…
5 Company Logos that you will probably never see again…
According to our article "10 years of Acquisitions in the Spine Business (2010-2019)" many acquisitions have taken place in the Spinal market in the last 10 years consolidating companies or assets, with an eye toward stimulating growth, gaining competitive advantages, increasing market share, or influencing supply chains. Many companies acquired, have disappeared for ever … [Read more...] about 5 Company Logos that you will probably never see again…
7 Signs That You Need To Fire Your Outside Product Development Firm
By Lawrence Binder--Chairman at Binder Biomedical, Inc. So, you finally got your medical device project started but you just can’t seem to cross the finish line. From failing prototypes, failing mechanical tests, going over budget, to slipping timelines… your medical device development firm is failing you. If you are an OEM, you start to wonder why you didn't do this project … [Read more...] about 7 Signs That You Need To Fire Your Outside Product Development Firm
Top 5 Mistakes Medical Device Inventors Make When They Have A New Idea
By Lawrence Binder/Chairman at Binder Biomedical, Inc. (linkedin.com)--The hardest part of developing a new idea is getting started. But getting started without considering this list could cause major project setbacks. Contact me to discuss how I can help navigate the complicated product development cycle and bring your new idea to life. List Below: Hiring an engineer or … [Read more...] about Top 5 Mistakes Medical Device Inventors Make When They Have A New Idea
Spine Business:Is the Past Better than the Present?
Is the past always better than the present? We love to reminisce about the good old days. . . When prices were high and the spine market was stable, growing and very profitable. Ten years ago, we did not have to deal with the pricing or with insurance companies pushing back harder than ever on spinal fusions, making it more difficult to gain approval for surgical procedures. … [Read more...] about Spine Business:Is the Past Better than the Present?