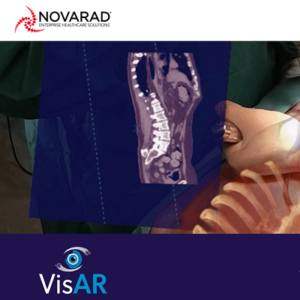
SALT LAKE CITY, June 15, 2022 /PRNewswire/ — VisAR, an augmented reality surgical navigation system from healthcare technology leader Novarad™, receives FDA 510(k) approval for precision guided intraoperative spine surgery. VisAR transforms a patient’s imaging data into a 3-dimensional hologram which is visible through an optical visor and superimposed onto the patient with submillimeter accuracy. This allows the […]
NEWS
What if innovation comes from leveraging the full potential of medical devices?
For years now, innovation efforts in spine surgery have been devoted to developing new treatments or finding new materials for implants. Little has been done to unleash the full potential of some of the most commonly used medical devices. Simple tools such as handle torques or surgical trays could however provide a wealth of information […]
Game-changing New Single-use Cervical and Mini-open Retractor to be Showcased at Becker’s Healthcare’s Spine, Orthopedic and Pain Management-Driven ASC Conference
SUNRISE, FL – On June 15, 2022, SURE Retractors Inc., a medical device company that has developed a range of industry-disruptive retractors for spinal surgery, announced it will be showcasing its new single- use Cervical and Mini-open Retractor at Becker’s Healthcare’s Spine, Orthopedic and Pain Management- Driven ASC Conference, which will take place June 16 […]
Xenco Medical Expands its Ambulatory Surgery Center Device Portfolio with FDA Clearance and Launch of its Multilevel CerviKit
SAN DIEGO–(BUSINESS WIRE)–Xenco Medical has expanded its ASC surgical device portfolio through the FDA clearance and launch of its Multilevel CerviKit™, an expansion of Xenco Medical’s breakthrough single-use cervical spine technology to include a comprehensive suite of implants and single-use instruments for 2, 3, and 4 level anterior cervical spine procedures. Optimized for the ambulatory […]
SI-BONE, Inc. Announces FDA Clearance for Expanded Indication of the iFuse-TORQ® Implant System
SANTA CLARA, Calif., June 13, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc. (SIBN), (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, today announced an FDA clearance for iFuse-TORQ® for pelvic fracture fixation, including acute, non-acute and non-traumatic fractures.* Fractures covered in this clearance include pelvic fragility fractures (fractures related […]
Accelus Announces Sales Leadership Appointments, Establishes U.S. East and West Sales Leaders
PALM BEACH GARDENS, Fla., June 13, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced enhancements to its sales team with the promotion of Lisa Jacobs to Vice President of Sales, Jim Fox to […]
Aurora Spine Corporation Issued Patent by USPTO For Its Interlaminar Single Implant Technology For Motion Preservation or Fusion
CARLSBAD, Calif., June 13, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of United States Patent No: 11,331,199 entitled “Spinal Implant for Motion Preservation or Fusion.” This patent covers Aurora’s […]
Historical Spine Systems (2): CSLP Cervical Plate
The first anterior cervical fusion surgeries were performed in the early 1950s by Bailey and Badgley. At that time, many others such as Cloward, Smith, and Robinson contributed greatly to the refinement of cervical fusion techniques. The high rates of nonunion and kyphosis rates in multi-level cases led to the design and development of another […]
Zavation Medical Products, LLC, receives FDA 510K Clearance for eZspand™ Lateral Cage as an addition to its eZspand™ Interbody System.
FLOWOOD, Miss., June 10, 2022 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the FDA 510K Clearance of eZspand™ Lateral, an expandable lumbar interbody fusion device. The eZspand™ Lateral, part of the Zavation eZspand™ Interbody System, features unmatched expandable […]
Surgical Robots in a Post-Pandemic Marketplace
(Globus Medical-2021)–Healthcare institutions continue to calculate the profound impact of the COVID-19 global pandemic.In a February 2021 American Hospital Association (AHA) report,1 research showed that 2021 hospital revenue estimated a $53 to 122 billion loss due to the lingering effects of COVID-19. This is already riding off the projected loss of $323.1 billion in 2020.2 As […]
SeaSpine Announces Full Commercial Launch of the WaveForm™ TO (TLIF Oblique) 3D-Printed Interbody System
CARLSBAD, Calif., June 08, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of its 3D-printed WaveForm TO (TLIF Oblique) Interbody System. WaveForm TO was designed for both PLIF (posterior lumbar interbody fusion) and […]
SpineGuard Announces a New Partnership with Sorbonne, CNRS and Inserm to associate its DSG® Technology With Ultrasound and Serve Surgical Robots
PARIS & BOULDER, Colo.—SpineGuard (FR0011464452 – ALSGD) (Paris:ALSGD), an innovative company that deploys its DSG (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announces the launch of a new three-year collaboration program with two labs of Sorbonne University CNRS and Inserm: the ISIR (Institute for intelligent systems and robotics) and […]
Aurora Spine Corporation Receives FDA 510(k) Clearance for DEXA SOLO-LTM Anterior Lumbar Interbody Fusion Device as part of its DEXA Technology Platform
CARLSBAD, Calif, June 06, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced that it had received FDA 510(k) clearance for its DEXA SOLO-L™ spinal fusion system. The 3D printed standalone anterior lumbar interbody […]
SMAIO Receives FDA 510(k) Clearance for Its Balance Analyzer 3D Surgery Planning Software
LYON, France–(BUSINESS WIRE)–Regulatory News: SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA, eligible for PEA-PME equity savings plans), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today announced that it has received FDA 510(k) clearance for its Balance Analyzer 3D […]
Six-Peat: CTL Amedica CTO Sean Suh Recognized as a Top Spine Inventor
DALLAS (PRWEB) JUNE 02, 2022–For the sixth year in a row, CTL Amedica Chief Technology Officer Sean Suh has been included in the Spinemarket Inc. Patent Analysis and Power Index, having been named to the current Top Twenty+ Inventors in Spine list. Suh has been included on the list every year, since its 2016 inception. This year, Suh […]
3Spine, Inc. Announces Medical Advisory Board Appointment
NASSAU, Bahamas, June 2, 2022 /PRNewswire/ — 3Spine, Inc., a U.S. medical device company developing total joint replacement for the lumbar spine, today announced the appointment of Pierce Nunley, MD, to the company’s Medical Advisory Board (MAB). The MAB appointment was made at the International Society for the Advancement of Spine Surgery (ISASS) meeting in the Bahamas. Dr. Nunley […]
Study Data Show Premia Spine’s TOPS™ Spinal Arthroplasty System Is Cost-effective Compared With TLIF, Becomes Dominant Strategy Over Time
NORWALK, Conn. – June 2, 2022 – Premia Spine, a medical technology company changing the way debilitating chronic leg and back pain is treated, today announced the publication of clinical data demonstrating that its TOPS System provides significant health system and societal benefit versus transforaminal lumbar interbody fusion (TLIF). The first and only facet joint […]
Clinical Trial to Test Replacement Devices for Two-Level Symptomatic Cervical Disc Disease
NEW YORK, June 1, 2022 /PRNewswire/ — A multicenter, prospective, randomized controlled trial is designed to assess the effectiveness of three different disc replacement devices used for two-level symptomatic cervical disc disease. The study is sponsored by Centinel Spine, LLC, and Hospital for Special Surgery (HSS) in New York City, is one of the centers participating in the research. Cervical […]
Launch of ART Fixation System from NGMedical, Germany
NONNWEILER, Germany–(BUSINESS WIRE)–NGMedical GmbH, a medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces the launch of its new ART® Fixation System in Europe. The ART® Fixation System was developed with the goal of providing the surgeon with a fixation system to perform dorsal spinal stabilizations simply, quickly and effectively. ART® […]
Camber Spine’s SPIRA-P and SPIRA-T Devices Enter Full National Launch Mode
KING OF PRUSSIA, PA — June 1, 2022 – Camber Spine, a leading innovator in spine and medical technologies has announced that it is entering into the next phase of the complete national launch of the SPIRA-P Posterior Lumbar Spacer and has recently commercialized its Spira-T Oblique Posterior Lumbar Spacer.Part of Camber’s SPIRA product platform, the SPIRA-P Posterior Lumbar Spacer is versatile […]
Stryker Gets FDA 510(k) Clearance for Q Guidance System
Stryker Corp. announced today that the Q Guidance System has received 510(k) clearance from the U.S. Food and Drug Administration. The company said its Q Guidance System, when used with the Spine Guidance Software, is an advanced planning and intraoperative guidance system designed to enable open or percutaneous computer-assisted surgery. Stryker said the Spine Guidance […]
SI-BONE, Inc. Receives FDA 510(k) Clearance for iFuse Bedrock Granite, a Breakthrough Pelvic Fixation and Fusion Technology
SANTA CLARA, Calif., May 31, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc., (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, announces FDA 510(k) premarket clearance of the iFuse Bedrock Granite Implant System (Granite). The Granite implant provides sacroiliac fusion and sacropelvic fixation as a foundational element for segmental […]
BIOMECH will be attending Global Spine Congress 2022.Join us in Las Vegas!
BIOMECH, the leading Orthopedic devices developer and manufacturer in Asia will be attending Global Spine Congress 2022. From June 2 to 4 in Las Vegas, BIOMECH will bring you the innovative spinal solutions including posterior spinal fixation system, Intervertebral Body Fusion and Interspinous Process Device (IPD), and more solutions for minimal invasive surgery. Please feel […]
4WEB Medical Announces Record First Quarter Performance
DALLAS, May 26, 2022 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced today that the company achieved record revenue in Q1 2022, largely driven by sales of the company’s stand alone devices for ALIF and ACDF procedures. “4WEB’s outstanding results […]
Medtronic reports full year and fourth quarter fiscal year 2022 financial results; announces 8% dividend increase
DUBLIN, May 26, 2022 /PRNewswire/ — Medtronic plc (NYSE: MDT) today announced financial results for its fourth quarter and fiscal year 2022, which ended April 29, 2022. Key Highlights FY22 revenue increased 5% reported and organic FY22 GAAP diluted EPS increased 40%; non-GAAP diluted EPS increased 26% FY22 cash flow from operations of $7.3 billion increased 18%; FY22 free cash flow […]
Peter Witke appointed Chair of Additive Surgical Pty Ltd
Adelaide, May 26 , 2022–Additive Surgical Pty Ltd today announced the appointment of Mr. Peter Witke as Chairman of its Board Of Directors. “With bringing the experience and skills set of this Dutch veteran in international sales and business development of surgical solutions, Additive Surgical feels excited about Peter to lead our board”, says Additive’s […]
Tsunami Medical and Additive Surgical announce strategic alliance
Modena / Adelaide, May 25th , 2022–Tsunami Medical Srl and Additive Surgical Pty Ltd today announced a strategic alliance with regards to both manufacturing and sales of 3D printed Spinal implant technology in Australia and New Zealand. This successful alliance started 18 months ago with a question which was as simple as meaningful: “what is […]