WINTER PARK, FLA. (PRWEB) SEPTEMBER 29, 2022–SpineNet LLC is pleased to announce that the SSP™ Anterior Cervical Plate System has received high marks during its initial validation series. The SSP™ requires only a single screw per vertebrae level and is available for multiple levels in an extensive range of sizes. The cost savings are immediate, eliminating one screw […]
NEWS
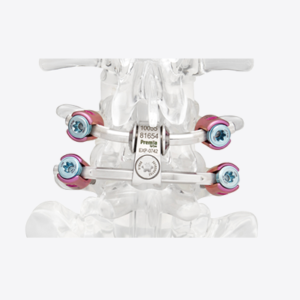
NuVasive Launches Reline Cervical for Posterior Cervical Fusion as Part of the C360 Portfolio
SAN DIEGO, Sept. 28, 2022 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, announced today the commercial launch of Reline® Cervical, a new fixation system for posterior cervical fusion (PCF), in targeted regions. “Reline Cervical completes the foundational three strategic pillars of C360™—anterior cervical decompression […]
NANISX LLC To Focus On ASC Spine Surgeries Hires Ex-Medtronic David Flickinger MBA As New CEO
MALDEN, MASS. (PRWEB) SEPTEMBER 27, 2022–Effective September 15, 2022 NANISX LLC, a spine medical device company excelling in developing less exposure surgery (LES) technologies in spine surgeries for ambulatory surgery centers (ASC) has announced that the firm has appointed former Medtronic, and Nuvasive sales executive, David Flickinger MBA as their new Chief Executive Officer (CEO). “I […]
SMAIO Announces Its First-half 2022 Results
Lyon (France), September 27, 2022 – 6 pm CEST – SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA, eligible for PEA-PME equity savings plans), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today published its […]
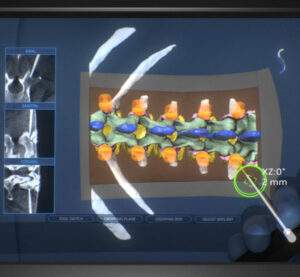
Stryker Launches Q Guidance System with Spine Guidance Software
KALAMAZOO, Mich.–(BUSINESS WIRE)–Stryker (NYSE:SYK), one of the world’s leading medical technology companies, announced today the launch of its Q Guidance System for spine applications. The System combines new optical tracking options provided by a redesigned, state-of-the-art camera with sophisticated algorithms of the newly launched Spine Guidance Software to deliver more surgical planning and navigation capability […]
Surgalign Launches Portfolio of Fortilink® with TiPlus™ Technology Products, Expanding its Addressable Market for Interbody Fusion Procedures
DEERFIELD, Ill., Sept. 26, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced the expansion of its Fortilink product portfolio with the introduction and commercial launch of a new family of interbody fusion devices […]
Safe Medical announces the renewal of its ISO 13485 certification of its production site on an extended perimeter including clean rooms
Eragny-sur-Oise, Fleurieux sur l’Arbresle, France, September 22th, 2022 5:45pm CET – Safe Medical , a subsidiary of Safe Group (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces the renewal of its iso 13485 certification for its industrial production […]
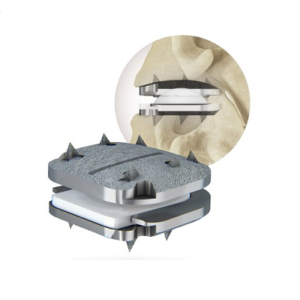
Centinel Spine® Announces First Commercial Use of the prodisc® C Vivo Cervical Total Disc Replacement System in the Western U.S.
WEST CHESTER, Pa., Sept. 21, 2022 /PRNewswire/ — Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) product in the Western U.S. In July, the company received U.S. Food and Drug Administration approval for 1-level […]
IMPLANET publishes its 2022 half-year results
Bordeaux, Boston, September 20, 2022 at 06:00 pm CEST – IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME plans), a medical technology company specializing in vertebral implants, announces its results for the first half of the current fiscal year, ended June 30, 2022, as approved by the Board of Directors on September 19, 2022. Ludovic Lastennet, […]
Stryker receives FDA clearance for OptaBlate™ Bone Tumor Ablation System
PORTAGE, Mich., USA, Sept. 20, 2022 /PRNewswire/ — Stryker ( NYSE:SYK), one of the world’s leading medical technology companies, announced today that its OptaBlate bone tumor ablation system (OptaBlate) received 510(k) clearance from the U.S. Food and Drug Administration. The addition of the OptaBlate technology to Stryker’s Interventional Spine (IVS) portfolio expands on its core competencies in vertebral […]
IMPLANET: Partnership With Sanyou Medical: Conditions Precedent Fulfilled
IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans) (Paris:ALIMP), a medical technology company specializing in vertebral implants, announces that all of the conditions precedent pertaining to the planned partnership with Sanyou Medical reported in the press release of June 29, 2022 have now been met. Implanet’s Board of Directors will meet shortly to determine […]
Elevation Spine, Inc. Closes $11 Million Series B Financing
MONTEREY, Calif.–(BUSINESS WIRE)–Elevation Spine, Inc., the leading innovator of integrated-fixation spinal technologies, today announced the completion of its Series B Preferred Stock financing, totaling $11 million. The funding was led by Technology Venture Partners (“TVP”) and included participation from Mutual Capital Partners (“MCP”) and existing investors. The company also announced the appointment of Donald Bossi […]
ulrich medical USA® Announces Corporate Relocation to Dallas-Fort Worth Metroplex
ST. LOUIS, Sept. 16, 2022 /PRNewswire/ — ulrich medical USA, Inc., a privately held medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, today announced it will relocate its US headquarters to Plano, Texas. Its current location is in Chesterfield, Missouri. “We believe it’s in the best strategic interest of the company to make this […]
Taiwan Food and Drug Administration Grants CTL Amedica Corp License to Market MATISSE™ ACIF Cage System
DALLAS (PRWEB) SEPTEMBER 15, 2022–The Taiwan Food and Drug Administration (TFDA), a part of the Ministry of Health and Welfare, has granted CTL Amedica Corporation a license for the MATISSE™ Anterior Cervical Interbody Fusion (ACIF) Cage System. The official license, DHA 05603556709, enables CTL Amedica to market the MATISSE™ ACIF Cage System in Taiwan, a crucial […]
SpineGuard Reports Six-Month 2022 Financial Results
SpineGuard (FR0011464452 – ALSGD) (Paris:ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today financial results for the half year ending June 30, 2022, as approved by the Board of Directors on September 15, 2022. Pierre JEROME, cofounder, Chairman & CEO of […]
Surgalign Announces Third Clinical Site in the Commercialization of its HOLO Portal™ Surgical Guidance System
DEERFIELD, Ill., Sept. 15, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced further advancement in the commercialization of its HOLO Portal™ surgical guidance system. The Company is pleased to report that Jaideep Chunduri, […]
Amplify Surgical®, Inc. Hosts Fall Spine Symposium with Cadaver Lab – featuring dualPortal™ endoscopic and dualX® system
IRVINE, CALIF. (PRWEB) SEPTEMBER 14, 2022–Amplify Surgical, Inc., a medical device company focused on innovative minimally invasive surgery for the lumbar spine, will be holding its Fall Spine Symposium, featuring dualPortal™ endoscopic and dualX® system, in New York City, New York on September 24, 2022. Amplify Surgical is partnering with expert surgeons from South Korea and […]
Dr. Kingsley R Chin Granted Patent To Add Wireless Sensor To AxioMed’s Total Disc Replacement To Target $23.50B Smart Medical Device Market
MALDEN, MASS. (PRWEB) SEPTEMBER 14, 2022 On September 29, 2017, Dr. Kingsley R Chin and engineers working at KICVentures Group quietly submitted their patent application to add remote sensing capabilities to their AxioMed viscoelastic total disc replacement. On September 8, 2020 they were awarded patent # US 10,765,527 B2. This was a seismic accomplishment since it […]
Mediliant Group Announces New Sales Director Nick Belloli
LE LOCLE, SWITZERLAND (PRWEB) SEPTEMBER 13, 2022–Leaders in medical device contract manufacturing, Mediliant Group, have announced the addition of sales expert Nick Belloli as the new Sales Director for the organization. The arrival of Belloli into this role plays a crucial part in Mediliant Group’s continued expansion as they work to enhance their capabilities to further […]
Omnia Medical and SpineGuard Announce a Strategic Alliance for Adult Spine Surgery in the United States
SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, and Omnia Medical, a medical device company focused on innovative solutions utilizing proven techniques, announced today the signature of a co-development and exclusive distribution agreement for adult spine surgery in the […]
Titan Medical Signs Definitive Agreement with Medtronic
TORONTO, Sept. 12, 2022 (GLOBE NEWSWIRE) — Titan Medical Inc. (Nasdaq: TMDI; TSX: TMD), a medical device company focused on the development and commercialization of innovative surgical technologies for single access robotic-assisted surgery (RAS), today announced the signing of a Definitive Agreement with Medtronic plc (“Medtronic”) (NYSE: MDT), a global leader in healthcare technology. The […]
Dr Kingsley R Chin steps down as CEO of NanoFuse Biologics LLC after merger with NANISX LLC
MALDEN, MASS. (PRWEB) SEPTEMBER 09, 2022–NanoFUSE Biologics is the world’s only FDA-approved synthetic fiber-based bioactive glass combined with demineralized bone matrix (DBM) for regenerative bone formation, promoting fusion and bone healing. NanoFUSE, when combined with an aqueous body fluid, forms a 3D ultra porous calcium hydroxyapatite layer for bone formation in as early as 24 hours. […]
SINTX Announces Succesful FDA 510(k) Pre-Submission Meeting for Silicon Nitride-Peek Composite Spine Implants
SALT LAKE CITY, Sept. 08, 2022 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (www.sintx.com) (NASDAQ: SINT) (“SINTX” or the “Company”), an original equipment manufacturer (OEM) of advanced ceramic materials for medical and technical applications, announced today it has held a successful 510(k) pre-submission meeting with the United States Food and Drug Administration (FDA) concerning the potential development […]
Surgalign to Exhibit at the Society for Minimally Invasive Spine Surgery (“SMISS”) Annual Forum and the North American Spine Society (“NASS”) 2022 Annual Meeting
DEERFIELD, Ill., Sept. 08, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced two upcoming industry events it will be participating in and showcasing many of its latest spine and technology innovations. The Society for […]
Innovative Spinal Biomaterial Demonstrates Early Healing in Retrospective Study
AUSTIN, Texas, Sept. 08, 2022 (GLOBE NEWSWIRE) — DiFusion, Inc., an Austin, Texas-based leader in biomaterial technological innovation focused on introducing bioactive polymers in spinal and orthopedic surgery, today announced the results of the study “Can a bioactive interbody device reduce the time horizon for Spinal fusions?” that validates ZFUZE as the first homogenous new […]
Centinel Spine® Announces First Commercial Use of prodisc® Cervical Total Disc Replacement Portfolio that Allows the Disc to be Matched to Patient Anatomy
WEST CHESTER, Pa., Sept. 7, 2022 /PRNewswire/ — Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) product. In July, the company received U.S. Food and Drug Administration approval for 1-level indications for prodisc C Vivo, […]
Premia Spine’s TOPS™ Spinal Joint Replacement Shows Potential to Relieve Back Pain and Maintain Mobility Without Adjacent Level Degeneration
NORWALK, Conn.–Premia Spine, a medical technology company changing the way debilitating chronic leg and back pain is treated, today announced that a single-center study evaluating its TOPS™ System for lumbar spinal stenosis and degenerative spondylolisthesis has been published in the journal Operative Neurosurgery. In the paper, “Mobility-Maintaining Arthroplasty of the Lumbar Spine with the Second-Generation TOPS […]