LEWISVILLE, TEXAS–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced results from a prospective, multicenter clinical study evaluating the safety and efficacy of the Trinity Elite™ cellular bone allograft (CBA) in lumbar spinal fusion procedures. Published in Neurology International, patients treated with the Trinity Elite CBA demonstrated fusion […]
NEWS
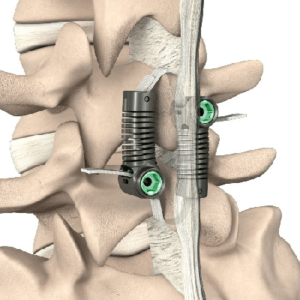
$12M Verdict Against DePuy Synthes for Spinal-Fusion Patent Infringement
On December 9, 2022, a Delaware federal jury found infringement by DePuy Synthes and awarded $12 million to RSB Spine. RSB Spine filed a lawsuit in 2019 in the US District Court for the District of Delaware for patent infringement based on DePuy’s manufacture and sale of various low-profile interbody plate spacer devices. Ultimately, the […]
Inspired Spine and IS Life are taking the next steps to educate the public through the Podcasts series “Essence of” available on youtube
BURNSVILLE, Minn., Dec. 20, 2022 /PRNewswire/ — In the last month, Inspired Spine CMO, Dr. Hamid Abbasi, with cooperation of IS Life social marketing director Amanda Armagost, have begun the podcast series, “Essence Of” with heavy emphasis on medicine to educate the public. The podcast airs LIVE on YouTube every Friday and focuses on education. Topics include, Medicine, Science, […]
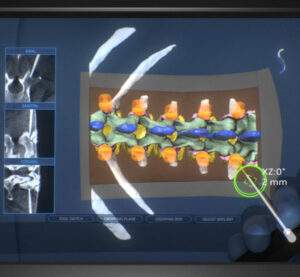
SURGLASSES received FDA 510(k) Clearance for Caduceus S
TAICHUNG, Taiwan, Dec. 19, 2022 /PRNewswire/ — SURGLASSES announced today that Caduceus S AR Spine Navigation System has received 510(k) clearance from the U.S. Food and Drug Administration and is ready to launch its products into the US market in the first quarter of 2023. Caduceus S is an augmented reality spine navigation system that will require only few-shots […]
BAGUERA® C and Navigated Instruments have received CE Approval under the MDR Regulation!
Spineart is thrilled to share a key milestone for the company; BAGUERA® C Cervical Disc Prosthesis, and our range of navigated instruments, are now CE Marked under the MDR 2017/745 (Medical Device Regulation) by the notified body TUV Sud. What is MDR and why does it matter?The MDR is the set of regulations that control […]
Empirical Spine Completes Full Premarket Approval (PMA) Submission to FDA for LimiFlex™ Dynamic Sagittal Tether™
SAN CARLOS, Calif., Dec. 19, 2022 /PRNewswire/ — Empirical Spine, Inc., a medical device company creating a new class of spinal implant, recently completed the final step in the U.S. Food & Drug Administration (FDA) submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year […]
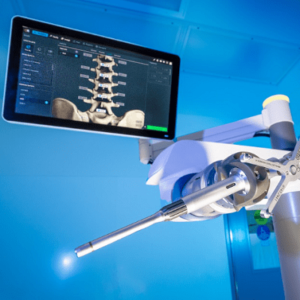
Spineart and eCential Robotics Announce a Partnership to Co-Develop Spine Surgery Applications and Co-Market the eCential Robotic’s Open Robotic Surgery Platform
Plan-les-Ouates (Geneva), Switzerland, and Gières (Grenoble), France, December 16, 2022 – Spineart, a leading provider of spine surgery implants and eCential Robotics, a growth MedTech company that designs and produces an open system unifying robotics, surgical navigation, and 2D/3D robotic imaging, today announced they entered a long-term collaboration agreement. The collaboration between the two companies […]
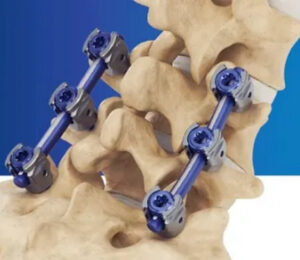
Surgalign Announces 100th Case Completed Utilizing its Recently Launched Cortera™ Spinal Fixation System
DEERFIELD, Ill., Dec. 15, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today announced a key milestone for its recently introduced posterior fixation platform – the Cortera™ Spinal Fixation System. Surgalign received FDA 510(k) in […]
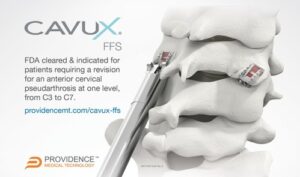
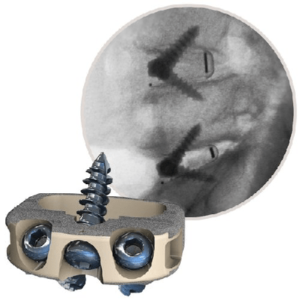
Providence Medical Technology announces FDA Clearance of the CAVUX® Facet Fixation System for the treatment of Cervical Pseudarthrosis
PLEASANTON, Calif., Dec. 15, 2022 /PRNewswire/ — Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® Facet Fixation System (FFS) for a new indication. The Facet Fixation System (FFS) is a novel integrated cage and screw […]
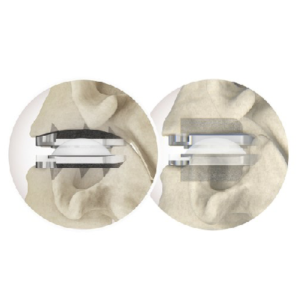
Life Spine Announces FDA 510(K) Clearance for the TruLift® Lateral Expandable Spacer System and Lateral Plate System
Huntley, IL, December 14th, 2022–Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the TruLift Lateral Expandable Spacer System and Lateral Plate System. Utilizing expandable technology and […]
Centinel Spine® Announces 250th Procedure with prodisc® C Vivo and prodisc® C SK Cervical Total Disc Replacement System
WEST CHESTER, Pa., Dec. 14, 2022 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the completion of the 250th procedure in the United States with its newly FDA-approved cervical total disc replacement system of prodisc solutions. This milestone, swiftly achieved since the Company announced […]
Surgalign™ Expands Adoption of its HOLO Portal™ Surgical Guidance System in Virginia
DEERFIELD, Ill., Dec. 13, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced additional commercialization of its HOLO Portal surgical guidance system. The Company is pleased to report that multiple cases have been successfully […]
150 U.S. Patients Treated in each of the One and Two-Level IDE Clinical Trials of the Spineart BAGUERA® C Cervical Disc Prosthesis
LAGUNA HILLS, CA, December 13th, 2022 – Spineart USA Inc. announced today that surgery has been performed on 150 patients in both the one and two-level for a total of over 300 patients to date in the U.S. Investigational Device Exemption (IDE) Clinical Trials of the BAGUERA® C Cervical Disc Prosthesis. The BAGUERA® C implant […]
The French Academy of Medicine grants the prestigious Charpak-Dubousset prize to research work for the prevention of bone breach in robotic surgery by the DSG® technology of SpineGuard
Paris (France) and Boulder (CO, USA), December 13th, 2022 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today that two researchers, the surgeon Elie Saghbiny and the engineer Jimmy Da Silva, were recognized for their collaborative work led […]
Surgeon Sentenced to 5 Years in Prison for Accepting Illicit Payments to Perform Spinal Surgeries at Corrupt Hospital
According to WorkersCompensation.com, yesterday, a neurosurgeon was sentenced to 60 months in federal prison for accepting approximately $3.3 million in bribes to perform spinal surgeries at a now-defunct Long Beach hospital. The facts occurred from 2010 to 2013, when the neurosurgeon accepted money from the owner in exchange for performing spinal surgeries at his hospital […]
(UPDATED 2022): +20 Expandable Lateral Cages to Know!
Since Nuvasive launched the XLIF procedure several years ago, this market segment has been growing. Today, most spinal companies have a lateral cage system in their portfolio. Technology and new materials have made it possible to develop more and more sophisticated implants that allow safer implantation and with less damage to the patient. The expandable technology has recently […]
17th German Spine Congress of 2022. Who are the 74 Exhibitors?
DWG 2022 will be held from December 7th to 10th in Berlin, Germany. The congress will take place at hub27 (Jafféstraße 2. 14055 Berlin). Below you can find the list of companies that exhibit there. To all of you who participate, we wish you a Great and Productive DWG 2022! EXHIBITORS DWG 2022: SIGNUS – the Sign for […]
HAPPE Spine Announces Andrew Iott as President and CEO
GRAND RAPIDS, Mich., Dec. 7, 2022 /PRNewswire/ — HAPPE Spine announces that Andrew Iott has accepted an offer to become the President and CEO. Mr. Iott brings over twenty years of experience in medical device product development and commercialization, with extensive knowledge of the spine market. As a co-founder of Globus Medical, Mr. Iott played a critical role in taking the […]
Pedicle Screw Insertion: Why take Unnecessary Risks when the Technology already exists?
In 1984, Airbus launched the A320, the first commercial jet to use a digital fly-by-wire control system. At its introduction, fly-by-wire and flight envelope protection was a new experience for many pilots. Until then, the pilot was in charge of handling the controls using all the instruments and levers. Now, fly-by-wire computers acted by stabilizing […]
How are the Top Spine Companies performing in 2022 so far?
Most companies have already published their nine months results (Q1+Q2+Q3). Which companies are growing in 2022? What have we seen in Q1,Q2,Q3 2022? The spine leading companies (Medtronic, Johnson&Johnson, Nuvasive, Stryker, Globus Medical, Zimvie, Seaspine, and ATEC Spine) have grown by 1,5%. Of the leading companies, Medtronic, Nuvasive, Globus Medical, Seaspine, and ATEC are growing […]
New Publication Supports Clinical Effectiveness of Centinel Spine’s Stand-Alone STALIF® M Integrated Interbody™ Portfolio
WEST CHESTER, Pa., Nov. 30, 2022 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the recent publication of a peer-reviewed study of its STALIF® M portfolio of products in the International Journal of Spine Surgery, titled: “Evaluation of Anterior Lumbar Interbody Fusion Performed Using […]
Biogennix’s DirectCell Advanced Bone Grafting System Used in 1000th Case
IRVINE, Calif. — November 29, 2022 — Irvine-based Biogennix, a market leader in advanced bone regeneration technology, announced today that its DirectCell Advanced Bone Grafting System has been used in more than 1000 cases as of this quarter. The DirectCell System includes an advanced synthetic bone graft material with properties that enhance cellular bone formation, along with novel instrumentation […]
Switzerland accepts medical devices with FDA approval
29.11.2022 (bioalps.org)– The Federal Council is to adapt national laws so that, in addition to medical devices with an EU certificate / CE mark, medical devices with U.S. Food & Drug Administration (FDA) approval will now also be recognised in Switzerland. Although not an EU member state or even part of the European Economic Area, […]
Eminent Spine’s ALIF Stand-Alone System Receives FDA 510(K) Clearance in October 2022
Plano, TX, November 08, 2022 –(PR.com)– Eminent Spine received 510(K) approval of their ALIF Stand-Alone System as of October 17, 2022. Eminent Spine will showcase the ALIF Stand-Alone System at the 50th CSRS conference in San Diego, CA on November 16 – 19, 2022. The ALIF implant was designed with the following: a tapered nose which […]
Medtronic reports second quarter fiscal 2023 financial results
DUBLIN, Nov. 22, 2022 /PRNewswire/ — Medtronic plc (NYSE:MDT) today announced financial results for its second quarter of fiscal year 2023, which ended October 28, 2022. Key Highlights Revenue of $7.6 billion decreased 3% as reported and increased 2% organic GAAP diluted EPS of $0.32 decreased 67%; non-GAAP diluted EPS of $1.30 decreased 2% Company updates guidance; expects organic revenue to accelerate in second […]
IMPLANET signs an exclusive distribution contract forSMTP Technology Co.’s ultrasonic surgical scalpel in France
Bordeaux, Boston, November 22, 2022 – 6:00 pm CET – IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral implants, today announces the signing of an agreement with SMTP Technology Co., a high-tech medical device company specialized in the manufacturing and marketing of ultrasonic medical equipment, for […]
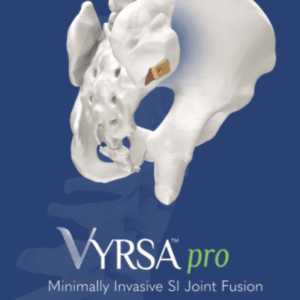
VYRSA Technologies, Inc. Appoints Earl R. Fender to the Corporate Board of Directors
KING OF PRUSSIA, Pa., Nov. 21, 2022 /PRNewswire/ — VYRSA Technologies, Inc., a specialty medical device company specializing in innovative sacroiliac fusion devices, announced today that, effective November 1, 2022, Earl R. Fender has been appointed to the Board of Directors. Fender previously served as the President & CEO of Nalu from 2020 to 2022 and as President & CEO […]