LARGO, FL (PRWEB) MAY 23, 2023–The patented, minimally invasive, SiJoin® T3 Transfixing Device is made from Ti-6AI-4V ELI titanium alloy using an additive manufacturing process, where the trabecular construction is engineered to mimic cancellous bone structure. The SiJoin® T3 Implant is engineered with a dual geometric shape designed to be implanted within the plane of the […]
NEWS
Join a major worldwide medical education event! Centinel Spine presents a live surgery and lumbar disc arthroplasty meeting broadcasting in real-time!
Join a major worldwide medical education event! Centinel Spine presents a live surgery and lumbar disc arthroplasty meeting broadcasting in real-time from Montpellier, France, May 25 & May 26, 2023. Learn more and get your personal zoom link by registering at: https://cvent.com/d/gkqpmy About Centinel Spine, LLC Centinel Spine®, LLC is a leading global medical device company addressing […]
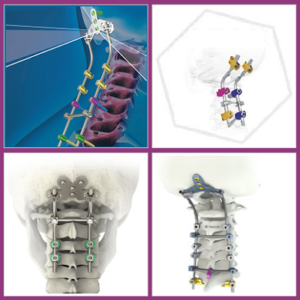
(UPDATED 2023): +65 Posterior Cervical Systems to Know!
The posterior cervical fusion is a type of cervical fusion surgery, that accounts the 15% to the cervical devices market share. The estimated market value of the posterior cervical fusion is $350M and it is a quite attractive niche for most of the Spine Companies which have already launched Posterior Cervical Systems. Although it is […]
Centinel Spine® Announces Record Worldwide Motion Preservation Revenue in First Quarter 2023
WEST CHESTER, Pa., May 18, 2023 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced record first quarter 2023 worldwide financial results in its Motion Preservation business. Record growth […]
FDA Awards CTL Amedica 510k Clearance for the NITRO Interbody Fusion Cage System Family
DALLAS (PRWEB) MAY 18, 2023—CTL Amedica Corporation has received 510k clearance from the U.S. Food and Drug Administration (FDA) to market the NITRO Interbody Fusion Cage System Family, designated K220513, made entirely of the fusion biomaterial silicon nitride. “We’re excited to announce the FDA 510k clearance to exclusively market the next generation of silicon nitride implants, […]
Xtant Medical Appoints Lori Mitchell-Keller to Board of Directors
BELGRADE, Mont., May 18, 2023 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the appointment of Lori Mitchell-Keller to the Company’s Board of Directors effective May 16, 2023. Ms. Mitchell-Keller will serve as a member of […]
We are proud to announce that Osimplant is Sponsor of SPINEMarketGroup in 2023!
Thank you Osimplant! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2023 through your PLATINUM sponsorship. About Osimplant Osimplant has been founded in Ankara in 2005 for providing the best spinal implants and services for healthcare professionals. Osimplant quality system has been built to fulfil compliance […]
SpineGuard and XinRong Medical expand their collaboration to deploy DSG® technology in China.
PARIS and BOULDER (CO), May 16, 2023, at 8:30 am CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, and XinRong Medical Group, a China leader in medical technology, announced today that they expanded their collaboration with the signature of three concomitant agreements: […]
Globus Medical Announces FDA Approval for its Non-Fusion Scoliosis Correction System, the Latest Advancement for Young Patients with Idiopathic Scoliosis
AUDUBON, Pa., May 15, 2023 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced the REFLECT™ Scoliosis Correction System has been granted approval from the US Food and Drug Administration, as the company’s first humanitarian device. REFLECT™ is designed to correct progressive scoliosis in young patients while preserving motion, maintaining stability, and allowing for […]
(UPDATED 2023): Argentine Spine Companies to Know…!
The nature of the public health system in Argentina encourages the participation of local companies who can provide devices at a lower price as a result of improved margins compared to foreign companies, who typically pay more for education, distribution and manufacturing. According to a report from iData Research, the total Argentinian spinal implants market […]
Intech Expands Manufacturing Capacity for Surgical Containers: ISO13485 Certified Facility Now Open in Rockaway, NJ
ROCKAWAY, N.J. (PRWEB) MAY 11, 2023–Intech, the market leader in the manufacturing of orthopedic medical devices, today announced that its new Intech Cases Inc. facility in Rockaway, New Jersey, has been awarded ISO13485 certification by AFNOR. Dedicated to the manufacturing of surgical cases, trays and caddies for the orthopedic industry, this 11,000 sq.ft. expansion allows Intech […]
Surgalign Announces First Quarter 2023 Results and Provides Update on its Business Operations
DEERFIELD, Ill., May 11, 2023 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today announced financial results for its 2023 first quarter ended March 31, 2023. 2023 First Quarter and Subsequent Corporate Highlights: “Throughout 2023, we have advanced our […]
Providence Medical Technology Announces Completion of Enrollment in the FUSE Clinical Study for High-Risk Cervical Fusion Patients
PLEASANTON, Calif., May 11, 2023 /PRNewswire/ — Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the close of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical […]
NuVasive Announces First Quarter 2023 Financial Results
SAN DIEGO, May 10, 2023 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced financial results for the quarter ended March 31, 2023. First Quarter 2023 Highlights “I am proud of our results and continued above-market growth,” said Chris Barry, chief executive officer of NuVasive. […]
Orthofix Reports First Quarter 2023 Results
LEWISVILLE, Texas–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX) today reported its financial results for the quarter ended March 31, 2023. Net sales were $175.2 million, earnings per share (“EPS”) was $(1.71), and adjusted EPS was $(0.10). “Orthofix delivered an exceptionally strong quarter with 12% year-over-year growth on a proforma basis and by minimizing any disruption associated with […]
Astura Medical Receives FDA 510(k) Clearance For The Sirion X Expandable Lateral Lumbar Interbody Fusion System
IRVING, TX – Astura Medical, a rapidly growing and innovative spine technology company, is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary Sirion X Expandable Lateral Lumbar Interbody Fusion (LLIF) System. Sirion X has been designed to deliver an all-encompassing range of expandable anatomic […]
Intelivation Technologies Launches Golden Isles™ Minimally Invasive Pedicle Screw System
PLYMOUTH MEETING, Pa., May 9, 2023 /PRNewswire/ — Intelivation Technologies, a medical device company with a cutting-edge orthopedic and spine implant product portfolio announced today that they have launched a new minimally invasive pedicle screw system. The Golden Isles™ Minimally Invasive Screw is a percutaneous system that increases visualization and procedural flexibility for surgeons, resulting in minimal soft tissue disruption for […]
Centinel Spine® to Present at 2023 Castellvi Spine Symposium
WEST CHESTER, Pa., May 9, 2023 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), will present at the 2023 Castellvi Spine Symposium, being held May 10-13 in Duck Key, Florida. On May 11, Ed […]
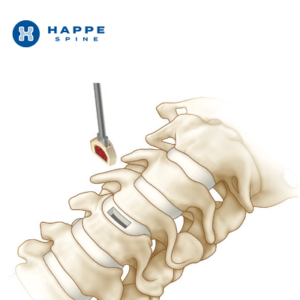
HAPPE Spine Announces FDA Clearance for the INTEGRATE-C™ Interbody Fusion System
GRAND RAPIDS, Mich., May 8, 2023 /PRNewswire/ — HAPPE Spine, a medical device company focused on bringing innovative materials to orthopaedic implants, announces that the company’s INTEGRATE-C™ Interbody Fusion System has received 510k clearance from the FDA. Powered by the HAPPE® platform, INTEGRATE-C™ is the first interbody fusion cage that is fully integrated with porosity and hydroxyapatite to provide a superior healing environment. […]
Surgalign Announces Release of HOLO™ AI Insights for Neurovascular Research
DEERFIELD, Ill., May 08, 2023 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, is proud to announce today, the release of HOLO™ AI Insights for use in neurovascular research. HOLO AI Insights, initially launched in March […]
Astura Medical Receives FDA 510(k) Clearance For Olympic Deformity’s Sublaminar Bands Supplemental System
IRVING, TX – May 4, 2023 –Astura Medical, a rapidly growing and innovative spine technology company, is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary Sublaminar Bands, which will serve as a supplementary set to the Olympic Deformity Posterior Spinal Deformity System. Building […]
Globus Medical Reports First Quarter 2023 Results
AUDUBON, Pa., May 04, 2023 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the quarter ended March 31, 2023. “Globus achieved a record quarter, delivering our highest quarterly revenue yet of $277 million, an increase of 21% over the first quarter of 2022 on a […]
ATEC Reports First Quarter 2023 Financial Results
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended March 31, 2023, and recent corporate highlights. Recent Highlights “We continue to execute against our mission to revolutionize the approach to spine surgery,” said Pat Miles, Chairman […]
OrthoPediatrics Corp. Announce Launch of RESPONSE™ Power Scoliosis System
WARSAW, Ind., May 04, 2023 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq: KIDS) a company focused exclusively on advancing the field of pediatric orthopedics, today announced the launch of their new Power System for the RESPONSE™ Scoliosis portfolio. The RESPONSE Power System is a complete portfolio of instruments for tapping pedicles, inserting screws, and […]
Xtant Medical Announces First Quarter 2023 Revenue Growth of 38% and Full Year 2023 Revenue Guidance
BELGRADE, Mont., May 04, 2023 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the first quarter ended March 31, 2023. The Company also introduced financial guidance for the full year 2023. […]
Orthofix STIM onTrack Recognized for Patient Engagement Innovation in 2023 MedTech Breakthrough Awards Program
LOS ANGELES (PRWEB) MAY 04, 2023–MedTech Breakthrough, an independent market intelligence organization that recognizes the top companies, technologies and products in the global health and medical technology market, today announced that Orthofix, a global medical device company with a spine and orthopedics focus, has been selected as winner of the “Best Patient Engagement Mobile App” award in […]
ZimVie Reports First Quarter 2023 Financial Results and Provides Update to Annual Guidance
WESTMINSTER, Colo., May 03, 2023 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today reported financial results for the first quarter ended March 31, 2023. Management will host a corresponding conference call today, May 3, 2023, at 4:30 p.m. Eastern Time. “I am pleased with […]