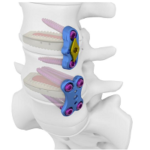
CEREBRO XLIF interbody fusion cage system is designed in a way to be implanted through a minimally invasive procedure from posterior lateral. The parts forming the system is manufactured from a Titanium alloy (Ti6Al4V – ELI) compliant with interbody application and from PEEK material which is a thermoplastic solution and which causes no artifact and compatible with MRI … [Read more...] about CEREBRO XLIF Cage system
LLIF
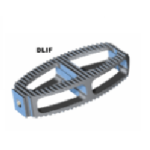
CLYDESDALE PTC
CLYDESDALE PTC Direct Lateral Interbody Fusion procedure provides spine surgeons with a complete minimally invasive solution for the treatment of degenerative lumbar conditions. By utilizing a direct lateral approach to the spine, this procedure enables placement of a large interbody graft into the disc space or anterior column support while avoiding the obstacles … [Read more...] about CLYDESDALE PTC
CASCADIA
CASCADIA™ Lateral Interbody System includes a full range of implant sizes carefully designed to accommodate the vertebral anatomy. This system is designed to work in conjunction with the RAVINE® Lateral Access System, offering a full line of instrumentation for the far lateral transpsoas approach. Lamellar Titanium Technology™ incorporates a porous structure along with rough … [Read more...] about CASCADIA
CONDUIT™ Lateral| EIT™ Cellular Titanium
3D printed cellular titanium implants that feature 80% porous macro-, micro- and nanostructures, are designed to mimic cortical and cancellous bone, and facilitate fusion. Conduit VIDEO ANIMATIONConduit-Interbody.Brochure-DepuySynthes.pdf Features: BONE-MIMICKINGCellular design facilitates fusion and mimics the properties ofbone with macro-, micro- and … [Read more...] about CONDUIT™ Lateral| EIT™ Cellular Titanium
CALIBER®-L
CALIBER®-L is an expandable lateral lumbar fusion device designed to streamline insertion and optimize fit. Insertion of CALIBER®-L is performed at a contracted height to ease insertion. Controlled distraction is designed to maximize indirect decompression through disc height restoration. Continuous expansion resists migration by optimizing fit. CALIBER-L VIDEO ANIMATION … [Read more...] about CALIBER®-L
MiRus 3DR LLIF
The 3DR™ Printed Lumbar Interbody Fusion System is indicated for intervertebral body fusion procedures in skeletally mature patients with degenerative disc disease (DDD) of the lumbar spine at one or two contiguous levels from L1-L2 to L5-S1. Features: 3D Pseudo Randomized (3DR™) Stochastic Titanium Lattice 600-1000μm Macro structure Large Bone Graft Windows Mimics … [Read more...] about MiRus 3DR LLIF
COUGAR® LS
The COUGAR® LS Lateral Cage System consists of carbon fiber reinforced PEEK (CFRP) cages. Benefits: About DePuy Synthes Founded 1895 in Warsaw, Indiana, by Revra DePuy, DePuy operates as a brand under the Johnson &Johnson Medical organization.On April 27, 2011, DePuy and Synthes agreed to a merger deal. The DePuy Synthes Companies offer the world’s most … [Read more...] about COUGAR® LS
Double Medical Lateral Interbody Fusion Cage
The OLIF cage is desgned with anatomical structure, large central window, pyramidal teeth, three lordotic angles, and could meet the special requirements of lumbar oblique lateral interbody fusion, focusing on curing degenerative disc diseases and spinal instabilities, revision procedures for post-discectomy syndrome, pseudoarthrosis or failed spondylodesis, degenerative … [Read more...] about Double Medical Lateral Interbody Fusion Cage
dualX™ Dual Expanding Interbody Fusion System
The dualX technology is comprised of a family of titanium expandable interbody devices designed to be implanted in lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) spinal procedures. The dualX portfolio contains varying footprints, heights and degrees of lordosis with post-expansion bone grafting … [Read more...] about dualX™ Dual Expanding Interbody Fusion System
Endoskeleton® TL
The Endoskeleton TL Interbody Fusion Device implants are available in a variety of lengths, widths, and heights for treatment in Lateral Lumbar Interbody Fusion and are designed with a large hollow region in the center to house autograft bone, allograft bone comprised of cancellous and/or corticocancellous bone, demineralized allograft with bone marrow aspirate, or a … [Read more...] about Endoskeleton® TL
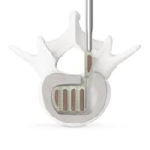
ELSA®
ELSA® is an expandable interbody fusion spacer with integrated fixation designed to maximize segmental lordosis while minimizing disruption to patient anatomy. ELSA VIDEO ANIMATION MARS™3V VIDEO ANIMATION MARS™ 3VL VIDEO ANIMATION Benefits: Adjustable Lordosis: Implants with adjustability from 5° to 20° or 15° to 30° allow for insertion at a smaller starting height … [Read more...] about ELSA®
ELSA®-ATP
ELSA®-ATP is an expandable lateral system designed to provide access to the lumbar spine anterior to the psoas muscle. Entering the disc space from this approach helps to avoid complications associated with the lateral trans-psoas approach and the lumbar plexus. This anterolateral working corridor also allows for direct access to L4‑L5 where a high iliac crest may complicate … [Read more...] about ELSA®-ATP
F3D Lateral
The F3D Lateral interbodies leverage the benefits of Mimetic Metal® technology which emulates key characteristics of natural bone to provide an optimal structure and environment for healing. Benefits: Optionally attach to the Oro™ Lateral PlateUnique trapezoidal geometry for precise anatomical fit100% open-pore architectureProvided sterile via … [Read more...] about F3D Lateral
IdentiTi™-LIF
ATEC’s IdentiTi Lateral Porous Ti Interbody Implants are designed to provide the biological, biomechanical, and imaging characteristics that surgeons seek in a fusion construct. The subtractive process used to manufacture each IdentiTi Implant results in more predictable mechanical performance and enhanced imaging characteristics. IdentiTi implants take advantage of bone’s … [Read more...] about IdentiTi™-LIF
InterContinental
InterContinental is the next generation system in minimally invasive lateral fixation. The plate and spacer are fully contained within the disc space, minimizing disruption to patient anatomy. The optimized screw design compressively loads the graft to help promote fusion. The system offers a wide variety of footprints in order to meet different patient anatomy and to ensure … [Read more...] about InterContinental
Imola Lateral IBF System
The Altus Spine Imola Lateral IBF System includes a unique, proprietary exposure method that allows for direct access and visualization of the surgical site. The innovative retractor blades convert directly from hand-held to table-mount stabilized via in situ retractor frame attachment without compromising the established access exposure. To ease implant insertion, the Imola … [Read more...] about Imola Lateral IBF System
IVA cage DLIF Titanium
About Dio Medical Dio Medical is a manufacturer of spinal Implants. Dio Medical has been in business since 2002 in South Korea and has been branched out to US Market. Our products are currently marketed internationally to Russia, China, Iran, Greece, Italy, Spain, Belgium, and Czecho. We are one of the leading company in spine industry and we are looking to grow aggressively … [Read more...] about IVA cage DLIF Titanium
InterFuse L Device
InterFuse L™ uses VTI’s patented modular insertion technique to provide a large lateral footprint through a significantly smaller lateral access channel. The InterFuse L™ alpha launch is scheduled for mid-November. The VTI InterFuse L device is an interbody fusion device that combines the large vertebral endplate contact area characteristic of many ALIF devices with the ability … [Read more...] about InterFuse L Device
InFill®
Infill® direct lateral lumbar accomplishes this through its patented shape and groundbreaking approach for graft delivery.The key to solid fusion is to optimize graft contact with the vertebral endplates. InFill VIDEO ANIMATION InFill Lateral-Brochure.Pinnacle.pdf Benefits: The Pinnacle Spine Infill® Lateral Interbody Device features a unique access port that … [Read more...] about InFill®