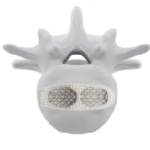
Dynam’X Ursus and related instruments set is designed for Ultra Lateral Interbody Fusion (ULIF) procedure. Dynam’X Ursus offers two implants’ options for open surgery and MIS surgery. Comprehensive range of sizes fits all anatomies. Large contact surface together with shell design concept is giving excellent combination of diagnostic compatibility (including MRi surveys) and … [Read more...] about Dynam’X Ursus
LLIF
Caprera
Caprera is a DLIF 3D Printed Titanium Cage with Expansion Feature. Caprera VIDEO ANIMATION Features: Excellent Stability: Additive manufacturing technology in combination with a unique geometrical implant design facilitates efficient and reliable primary and secondary fixation.Wide Variety of Footprints, Angles & Heights : The unique “net” structure, with its pore … [Read more...] about Caprera
Giannutri
Giannutri es a DLIF 3D Printed Titanium Cage .Expandable.The design concept of Giannutri, 3D printed DLIF cage, is made to meet well experienced surgeons’ requirements. Tsunami Medical offers implants in a wide range of footprints, heights, and lordosis angles, providing just one system matching patients’ natural anatomy and surgeons’ preferences. For Giannutri, Tsunami … [Read more...] about Giannutri
Idys®-LLIF 3DTi
Idys LLIF 3DTi is the Lateral system developed by Clariance Spine that incorporates the latest technology with the following Features: STRONG FIXATIONConcave plate design with a 2-screw configuration provides a wide range of angulation for optimal cortico-cancellous bone purchase. EASY SCREW INSERTIONUnique plate design facilitates an extensive angulation range for easy … [Read more...] about Idys®-LLIF 3DTi
Stromboli
The new XLIF cages are developed after an extended study on the anatomical shape of the spine in the lumbar segment.It’s anatomical shape, with a “bone like surface”guarantee perfect stability and a real stand alone solution.The bone grows inside the cage filling the entire porous structure , the cage can be easily filed with bone substitute to speed up the integration of the … [Read more...] about Stromboli
Aries-L
The Aries-L interbody fusion device features a proprietary multi-axis mesh and optimized micro-surface topology, both of which are designed to facilitate fusion. The product’s lattice helps increase the implant’s porosity to 80%, which provides unparalleled in-situ radiovisibility compared with other titanium implants. The implant’s anatomic profile, anti-migrational teeth, and … [Read more...] about Aries-L
Aditus Lumbar Xlif Cage
TheAditus Lumbar XLIF cage is a Lateral cage with an anatomical design, manufactured in PEEK and Titanium Alloy. Features: Anatomical DesignPEEK Material and TI-6AL-4V ELI ( ISO 5832-3 )Titanium Alloy MaterialTitanium Pin Indicators for Radiological ImagingSpecial Thread Structure Providing Strong Adhesion at Surfaces of its EndplatesEasy to Place within Disc Distance and … [Read more...] about Aditus Lumbar Xlif Cage
AnyPlus Lateral
The AnyPlus® Direct Lateral Interbody Fusion implant is manufactured from PEEK OPTIMA® and is designed with a tapered leading edge for easy insertion to provide optimal intervertebral fit and ample graft cavity to promote bony ingrowth. Specifically designed to complement the implant, the GS Retractor allows for tissue retraction and implant delivery in one. About GS … [Read more...] about AnyPlus Lateral
ADONIS®-LLIF
ADONIS®-LLIF cages are indicated for lumbar and lumbosacral interbody fusion via a direct lateral approach. The direct lateral approach is a minimally invasive approach, avoiding direct exposure of the anterior vessels and posterior nerve and bone structures. The implants have been designed to adapt to the anatomy of the vertebral bodies in order to reliably restore the … [Read more...] about ADONIS®-LLIF
Aero-LL
Aero-LL is a Lateral Lumbar Interbody and Fixation System that features Aerofoil™ Compression Technology, the only in-line LLIF (lateral lumbar interbody fusion) device to offer compression across the disc space. Aero-LL-SGT-Stryker.pdf Shaped like inverted plane wings, Aero-LL’s unique anchors are designed to pull the vertebral bodies towards the implant as they are … [Read more...] about Aero-LL
AVS® Aria™ PEEK Spacer System
ARIA is a lateral approach system including access, instrumentation, and interbody.The ARIA was designed to provide access and treatment to the lumbar spine via a lateral or anterolateral approach, including anterior retroperitoneal exposure through a small incision. AVS-ARIA-Lateral-Brochure-Stryker.pdf Benefits: Open IOM strategy with integrated components Retractor … [Read more...] about AVS® Aria™ PEEK Spacer System
Abacus Lateral Spacer System
The Abacus® Lateral Spacer System is available in multiple sizes with a biconvex implant shape designed to provide conformance to patient anatomy/pathology. The Abacus® Lateral Spacer System is intended to be introduced to the disc space via the lateral approach. Optimal implant fit: Attributes of Abacus® Lateral Spacer System: About Spine Wave Spine Wave's … [Read more...] about Abacus Lateral Spacer System
ALEUTIAN®
ALEUTIAN® has been designed to work in concert with the RAVINE® Lateral Access System, provides anterior column support, bridging the disc space, and includes a full line of instrumentation designed specifically for the far lateral transpsoas approach.The Aleutian Lateral Interbody System is designed to work in concert with the Ravine or Niagara Lateral Access Systems. … [Read more...] about ALEUTIAN®
Avenue® L
Avenue® L Lateral Lumbar Cage is designed for strength, versatility, and stability. It represents the pinnacle of lateral lumbar cage evolution incorporating zero-profile, intradiscal, and integrated fixation. The enhanced in-line, self-guided VerteBRIDGE® Plating Technology facilitates simplified cage insertion all through a direct, minimally invasive … [Read more...] about Avenue® L
AccelFix-XTP
AccelFix-XTP is an expandable cage specifically designed for the ATP approach.AccelFix-XTP Expandable Lateral Lumbar Cage System is composed of titanium alloy lateral cages which helps to restore disc height, segmental lordosis, and stability, while offering a customized fit for each patient’s unique anatomy. AccelFix-XTP VIDEO ANIMATION Features & … [Read more...] about AccelFix-XTP
AccelFix-XL
The AccelFix-XL expandable cage system for lateral approach helps restore disc height, segmental lordosis, and stability while offering a customized fit for the patient’s unique anatomy. AccelFix-XL VIDEO ANIMATION Features & Benefits Large graft window allows for greater graft volume to enhance fusion potentialSterile packaged implants help reduce surgical site … [Read more...] about AccelFix-XL
Battalion Lateral System
Battalion Lateral System with the Squadron Lateral Retractor provides surgeons with a next-generation lateral system with unrivaled, unique functionality designed to improve clinical outcomes by reducing tissue creep, minimizing psoas retraction time, and achieving alignment and fusion objectives. Situation : OUT OF THE MARKET (Replaced by the IdentiTi system) The system … [Read more...] about Battalion Lateral System
Calibrate LTXTM lateral expandable implant system
The Calibrate LTXTM is a lateral expandable implant system, technology designed to better achieve alignment goals with precise lordosis control and disc height restoration.The Calibrate LTX expandable lateral system seamlessly integrates with ATEC's entire lateral platform. The simplicity and ease of insertion of the implant is unique compared to the over-engineering that … [Read more...] about Calibrate LTXTM lateral expandable implant system
CoRoent XL
CoRoent XL is indicated for intervertebral body fusion of the spine in skeletally mature patients. The System is designed for use with autogenous bone graft to facilitate fusion.The CoRoent XL Interfixated System is intended for use at either one level or two contiguous levels in the lumbar spine, from L2 to L5, for the treatment of degenerative disc disease (DDD) with up to … [Read more...] about CoRoent XL