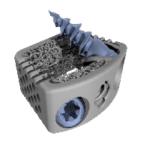
The Hexanium ACIF cage is a 3D printed standalone cervical interbody system made of titanium. It combines titanium rough surface, optimized porosity and honeycomb structure. The user-friendly one-step locking mechanism was developed to make the surgery as fast as possible. Benefits: About SpineVision Since its creation SpineVision has played a pioneering role in … [Read more...] about Hexanium ACIF cage
ACIF
HRCC®
Locking cervical cage HRCC® is part of a range of stand alone cages offering a primary and secondary stability locking mechanism without the need of anterior plate fixation (please refer to instructional notice of HRCC®implants.The cage is inserted according to routine technique, then the surgeon locks the cage by simple rotation of the instrument.The blade penetrates into … [Read more...] about HRCC®
HiveTM C
Utilizing high-definition 3D printing, HiveTM Interbody Fusion Devices all feature a Soft Titanium® lattice core with HD’s 4 evolutionary technologies. The HiveTM family includes multiple footprint and lordosis options for each surgical approach. Hive Cervical-Brochure.HD LifeSciences.pdf Benefits: Reduced Stiffness: HiveTM interbodies using Soft Titanium® technology … [Read more...] about HiveTM C
HEDRON C™ 3D Printed
HEDRON C™ is a 3D printed anterior cervical interbody fusion spacer that features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant. HEDRON Cervical VIDEO ANIMATION Features: The Face of Fusion:An ovine interbody study demonstrated significantly more bone ingrowth within HEDRON™ implants at 6 weeks post-op compared to … [Read more...] about HEDRON C™ 3D Printed
HEDRON IC™
HEDRON IC™ is a 3D printed ACDF spacer that can be used as an interbody spacer itself or combined with the COALITION AGX Plate and screws as a stand-alone plate-spacer. HEDRON IC™ features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant. Features: The Face of Fusion:An ovine interbody study demonstrated significantly more … [Read more...] about HEDRON IC™
Gemini™-C
The Gemini™-C Cervical Interbody’s proprietary hybrid design capitalizes on the osteoconductive properties of rough, porous titanium, while allowing for the radiolucent and biomechanical properties of PEEK. The anatomical footprint and domed profile mimic the shape and concavity of the disc space, allowing for maximum endplate coverage. By addressing the clinical needs of … [Read more...] about Gemini™-C
Intervertebral cervical locking cage
Intervertebral cervical locking cages, made of highly biocompatible materials (PEEK polymer, titanium alloy and tantalum) are used in C3 – C7 spine spondylodesis.Implants are designed so, to match the anatomy of cervical vertebrae the best, to ensure maximum safety of their use. Large spaces inside the cage allow for application of autologous bone graft or bone substitute. The … [Read more...] about Intervertebral cervical locking cage
F3D C2 Stand Alone Cervical
The F3D-C2 Stand-alone Cervical System is an interbody fusion system comprised of a spacer with two integrated bone screws secured by a locking mechanism within the cage. F3D C2 Stand ALONE Cervical VIDEO ANIMATION Benefits: Streamlined construct features biomechanical benefits of Mimetic Metal® 3D printing technologyScrews anchor through zero-step anti-back-out … [Read more...] about F3D C2 Stand Alone Cervical
GENOSS 3d Cage™ Cervical Cage
A cage-like agglutinating prosthesis used to treat structural anomalies caused by degenerative disc herniation of the cervical spine during an orthopedic or neurosurgery procedure, GENOSS 3d Cage™ Cervical Cage is a spinal cage that is transplanted between bones or artificial bone implants in order to provide sufficient space for mechanic safety or interspinous agglutination. … [Read more...] about GENOSS 3d Cage™ Cervical Cage
Idys®-C ZP 3DTi
Idys-C ZP 3DTi is Clariance’s new ZERO PROFILE CERVICAL CAGE. Benefits: ANATOMICAL SHAPEThe anatomical shape of the cage conforms to the patient’s cervical vertebrae for enhanced stability. ZERO PROFILEZero anterior profile to avoid any interference with the direct environment. SECURED CAGETwo integrated bone screws secured by a locking mechanism. ERGONOMIC … [Read more...] about Idys®-C ZP 3DTi
Irix-C™
Irix-C™is a standalone low profile anterior cervical integrated fusion system featuring titanium plasma-coated teeth and two locking screws protected by a resilient locking arm mechanism. The Irix-C Cervical Integrated Fusion System consists of an integrated endoskeleton, surrounded by an outer PEEK ring and two screws. It is intended for spinal fusion procedures at one level … [Read more...] about Irix-C™
IdentiTi™ C
ATEC’s IdentiTi Cervical Porous Ti Interbody Implants are designed to provide the biological, biomechanical, and imaging characteristics that surgeons seek in a fusion construct. The subtractive process used to manufacture each IdentiTi Implant results in more predictable mechanical performance and enhanced imaging characteristics. IdentiTi implants take advantage of bone’s … [Read more...] about IdentiTi™ C
InterPlate™ IFD
The InterPlate™ IFD is indicated for intervertebral body fusion of the spine in skeletallymature patients. The device system is designed for use with autograft to facilitate fusion.One device is used per intervertebral space. Benefits: Provides the benefits of an interbody spacer and an anterior cervical plate, without any of the drawbacks Combined fixation plate and … [Read more...] about InterPlate™ IFD
IN:C2 Cervical Cage
IN:C2 cervical cage is to be used during spinal fusion. IN:C2 serves to stabilize the spine while bony fusion develops.The IN:C2 System consists of a 'U' shaped PEEK block in multiple footprint configurations, heights and lordosis angles. The PEEK implants contain a titanium marker intended to verify position radiologically. The IN:C2 is a stand-alone system, intended for use … [Read more...] about IN:C2 Cervical Cage
Kentro SELF Standalone Cervical cage
Kentro SELF Standalone cage is used in traditional ACDF procedure to provide biomechanical strength for the cervical region by minimizing the disruption in patient anatomy. Its design combines the functionality of a cervical interbody spacer and benefits of an anterior cervical plate. Features About Rivarp Medical Rivarp Medical, is a Bangalore based medical devices … [Read more...] about Kentro SELF Standalone Cervical cage
Latitude-C™ Porous Ti Cervical Interbody Spacer
Latitude-C™ Porous Ti Cervical Interbody Spacer is designed to consider the uncinate process of the cervical vertebra. The lateral angled shape is a shift from traditional rectangular shape and mimics patient anatomy. The variety of profiles and angles provided allows a perfect fit at every fusion level and match individual patient anatomy. The Latitude-C™ Porous Ti Spacers … [Read more...] about Latitude-C™ Porous Ti Cervical Interbody Spacer
LorX ACIF Peek Cage
LorX ACIF Peek Cage family have an original design in various heights to restore the interbody space.Specially designed (patent pending) titanium blade, positioned in the middle of the cage body, for the perfect fixation. There is no need for extra fixation implants like plates etc.Two adequate graft spaces for enhanced bone fusion. Benefits: Better Stability The special … [Read more...] about LorX ACIF Peek Cage
L-ACIF
L-ACIF, is a Cervical cage with locking screws manufactured by the Company Ecosintesis from Colombia. Benefits: Concavity back side. Two titanium markers to effectively locate the implant position during radiological examination. Central space to fill with bone graft substitute. Side slits to promote ossification inside the implant. Non-slip serrated surfaces. Convergent … [Read more...] about L-ACIF
LONESTAR® Cervical Stand Alone
The LONESTAR CSA implant is a stand-alone spacer system designed to provide the biomechanical strength to a traditional or minimal invasive ACDF procedure with less disruption of patient anatomy and preserve the anatomical profile. The LONESTAR Cervical Stand Alone system helps to preserve the natural sagittal anatomic profile of the cervical spine while providing anterior … [Read more...] about LONESTAR® Cervical Stand Alone