Kalamazoo, Michigan, July 30, 2020 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) reported operating results for the second quarter of 2020:
The response to the COVID-19 pandemic has included unprecedented measures to slow the spread of the virus taken by local governments and health care authorities globally, including the postponement of deferrable medical procedures and social contact restrictions, which have had a significant negative impact on Stryker’s operations and financial results.
Second Quarter Results
- Reported net sales decreased 24.3% to $2.8 billion
- Organic net sales decreased 24.0%
- Reported operating loss margin of (0.7%)
- Adjusted operating income margin(1) of 12.5%
- Reported EPS decreased 117.5% to ($0.22)
- Adjusted EPS(1) decreased 67.7% to $0.64
“Our second quarter results were negatively impacted by COVID-19, but I am pleased with the resiliency and creativity that our team displayed in supporting our customers and continuing to advance our new product pipelines,” said Kevin A. Lobo, Chairman and Chief Executive Officer. “We were encouraged to see increased sales momentum through the quarter and into July and are poised to capitalize on the broader resumption of deferrable surgeries.”
Sales Analysis
Consolidated net sales were significantly negatively impacted by the global response to the COVID-19 pandemic, resulting in lower unit volume across all segments.
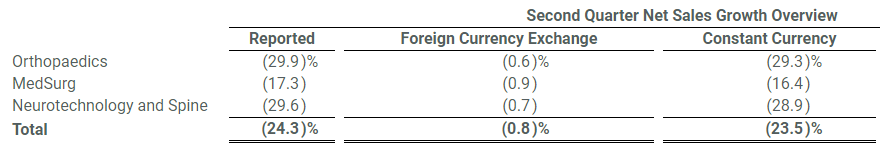
Consolidated net sales of $2.8 billion decreased 24.3% in the quarter and 23.5% in constant currency. Organic net sales decreased 24.0% in the quarter including 23.8% from decreased unit volume and 0.2% from lower prices.
Orthopaedics net sales of $0.9 billion decreased 29.9% in the quarter and 29.3% in constant currency. Organic net sales decreased 29.3% in the quarter including 28.1% from decreased unit volume and 1.2% from lower prices.
MedSurg net sales of $1.3 billion decreased 17.3% in the quarter and 16.4% in constant currency. Organic net sales decreased 17.0% in the quarter including 17.2% from decreased unit volume partially offset by 0.2% from higher prices.
Neurotechnology and Spine net sales of $0.5 billion decreased 29.6% in the quarter and 28.9% in constant currency. Organic net sales decreased 29.9% in the quarter including 30.4% from decreased unit volume partially offset by 0.5% from higher prices.
Earnings Analysis
Earnings were significantly negatively impacted by the global response to the COVID-19 pandemic. Reported net earnings (loss) of ($83) million decreased 117.3% in the quarter, including $170 million of charges related to certain in-process asset impairments and product line and other exit costs resulting from our decision to suspend certain investments due to pandemic-related constraints. Reported net earnings (loss) per diluted share of ($0.22) decreased 117.5% in the quarter. Reported gross profit margin and reported operating income (loss) margin were 56.0% and (0.7%) in the quarter. Adjusted gross profit margin(1) and adjusted operating income margin(1) were 57.3% and 12.5%. Adjusted net earnings(1) of $245 million decreased 67.4% in the quarter. Adjusted net earnings per diluted share(1) of $0.64 decreased 67.7% in the quarter.
2020 Outlook
The global response to the COVID-19 pandemic has had, and we expect will continue to have, a significant negative impact on Stryker’s operations and financial results. While we reported overall decreased unit volume in the quarter, most of our businesses saw gradual recoveries in the month of June 2020. Due to the uncertain scope and duration of the pandemic, and uncertain timing of global recovery and economic normalization, we are unable to estimate the overall impacts on our operations and financial results, which could be material. Accordingly, we will not be providing third quarter or full-year organic sales growth or earnings guidance for 2020.
(1) A reconciliation of the non-GAAP financial measures: adjusted gross profit margin, adjusted operating income and adjusted operating income margin, adjusted net earnings and adjusted net earnings per diluted share, to the most directly comparable GAAP measures: gross profit margin, operating income (loss) and operating income (loss) margin, net earnings (loss) and net earnings (loss) per diluted share, and other important information accompanies this press release.
Conference Call on Thursday, July 30, 2020
As previously announced, Stryker will host a conference call on Thursday, July 30, 2020 at 4:30 p.m., Eastern Time, to discuss the company’s operating results for the quarter ended June 30, 2020 and provide an operational update.
To participate in the conference call dial (877) 702-4565 (domestic) or (647) 689-5532 (international) and be prepared to provide conference ID number 1335868 to the operator.
A simultaneous webcast of the call will be accessible via the company’s website at www.stryker.com. The call will be archived on the Investor Relations page of this site.
A recording of the call will also be available from 8:00 p.m., Eastern Time, on Thursday, July 30, 2020, until 11:59 p.m., Eastern Time, on Friday, August 7, 2020. To hear this recording, you may dial (800) 585-8367 (domestic) or (416) 621-4642 (international) and enter conference ID number 1335868.
Caution Concerning Forward-Looking Statements
This press release contains information that includes or is based on forward-looking statements within the meaning of the federal securities laws that are subject to various risks and uncertainties that could cause our actual results to differ materially from those expressed or implied in such statements. Such factors include, but are not limited to: the impact on our operations and financial results of the COVID-19 pandemic and any related policies and actions by governments or other third parties; the failure to satisfy any of the closing conditions to the acquisition of Wright Medical Group N.V. (“Wright”), including the receipt of any required regulatory clearances (and the risk that such clearances may result in the imposition of conditions that could adversely affect the expected benefits of the transaction); timing of the closing of the acquisition of Wright; unexpected liabilities, costs, charges or expenses in connection with the acquisition of Wright; the effects of the proposed Wright transaction (or the announcement thereof) on the parties’ relationships with employees, customers, other business partners or governmental entities; weakening of economic conditions that could adversely affect the level of demand for our products; pricing pressures generally, including cost-containment measures that could adversely affect the price of or demand for our products; changes in foreign exchange markets; legislative and regulatory actions; unanticipated issues arising in connection with clinical studies and otherwise that affect U.S. Food and Drug Administration approval of new products, including Wright products; potential supply disruptions; changes in reimbursement levels from third-party payors; a significant increase in product liability claims; the ultimate total cost with respect to recall-related matters; the impact of investigative and legal proceedings and compliance risks; resolution of tax audits; the impact of the federal legislation to reform the United States healthcare system; costs to comply with medical device regulations; changes in financial markets; changes in the competitive environment; our ability to integrate and realize the anticipated benefits of acquisitions in full or at all or within the expected timeframes, including the acquisition of Wright; and our ability to realize anticipated cost savings. Additional information concerning these and other factors is contained in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. We disclaim any intention or obligation to publicly update or revise any forward-looking statement to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that affect the likelihood that actual results will differ from those contained in the forward-looking statements.
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. More information is available at www.stryker.com.
For investor inquiries please contact:
Preston Wells, Vice President, Investor Relations at 269-385-2600 or [email protected]
For media inquiries please contact:
Yin Becker, Vice President, Communications, Public Affairs and Corporate Marketing at 269-385-2600 or [email protected]
