PARIS and BOULDER (CO), July 9, 2020 – 08:00am CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today that its first half 2020 revenue was € 2.3 M.
Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, declared: “As all the orthopedic industry, SpineGuard has significantly been impacted by the COVID-19 pandemic since mid-March. During this particularly challenging period, the entire team has demonstrated strong resilience and we managed to secure our cash runway thanks to a tight control of the company’s expenses, a renewed equity line agreement and under the protection of the voluntarily filed ‘sauvegarde’ procedure in France and Chapter 11 in the USA. Sales volumes went back up these last weeks with the gradual resuming of elective surgeries. The context remains uncertain but feedbacks from the first surgeries performed with our new DSG Connect platform and the ongoing discussions with potential strategic partners leave us with interesting prospects.“
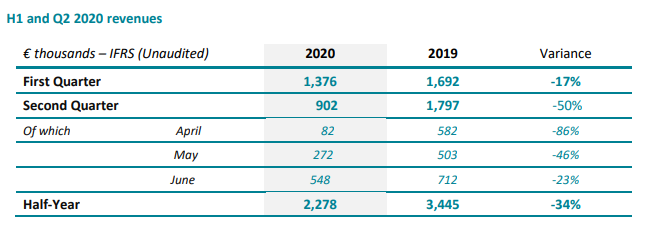
In the United States, the revenue of the second quarter 2020 decreased by 50% to $ 862 K from $ 1,688 K. Volumes were significantly impacted by COVID-19 since mid-March as elective surgeries were postponed. The activity was close to zero in April, resumed in May and accelerated in June. The momentum appears to continue early July. Despite the resurgence of the pandemic in several states, particularly in the south of the country and the fear of certain patients of contracting the virus at the hospital, financial analysts covering our sector such as Michael Matson (Needham), Kyle Rose (Canaccord Genuity) or Ryan Zimmerman (BTIG) anticipate the continuation of the resumption of elective surgeries and a gradual catching up of postponed cases, most of the patients affected by these postponements being in great pain.
In the rest of the world, all areas are affected by the pandemic with time lags. Like the United States, Europe has rebounded in recent weeks while the situation remains difficult in Latin America and Asia.
2,422 DSG units were sold in the first half of 2020 (from 3,875 units in 2019) of which 1,508 units (62%) were sold in the United States.
Status of the French ‘Sauvegarde’ and Chapter 11 procedures
Assisted by the French Trustee (‘administrateur’) Maître Thévenot, the Company is currently drafting its safeguard plan and, depending on the hearing schedule, should be able to present it by the end of September. This presentation must be made in coordination with the American procedure which already had several hearings in March and June. This phase will enable to formalize a proposal for debt restructuring.
SpineGuard is therefore continuing its discussions with its main creditors to arrange the “venture” debt and the FEI Innovation loan respectively. The current procedure does not exclude the possibility of a pre-packed agreement which would shorten it.
Pending the finalization of the safeguard plan, the payment of debts prior to the opening of the procedure is frozen. It should also be remembered that the safeguard procedure freezes any ability to exercise existing securities or collateral. As a reminder, as at January 31, 2020, the outstanding capital amounted to € 3.8 M compared to € 4.5 M at the conclusion of the ‘venture’ bond debt and of € 0.9 M against € 1.5 M for the FEI Innovation loan.
PERSPECTIVES
The cash position as of June 30, 2020 of € 1.2 M plus the secured equity line facility for € 2.4 M means that the total cash available to the Company is € 3.6 M. Considering the current cash position, the secured convertible bond facility and the recurring expected business, the Company estimates that it can fund its needs until mid-2022.
The Company remains focused on the following objectives for the second half of 2020:
- Continue the pre-launch in Europe of the new generation of PediGuard equipped with the DSG-Connect module, a wireless interface which adds the visual signal to the audio to optimize signal processing, allowing data recording as forensic evidence perform clinical studies on bone quality.
- Obtain DSG-Connect US clearance and initiate its pre-launch there before the end of 2020.
- Continue to provide scientific evidence of the value of DSG for surgical robotics.
- Maintain, as much as possible, the course of operational profitability in the context of COVID 19.
- Intensify collaboration with ConfiDent ABC on dental applications with the co-development of a new generation of products incorporating DSG technology.
- Close other industrial and strategic partnerships, in particular for the robotic application, assisted by the investment bank Healthios Capital Markets.
Next financial press release: 2020 Half-year financial results on September 15, 2020
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices that have been used in over 75,000 surgical procedures worldwide. Fifteen studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these solid fundamentals and several strategic partnerships, SpineGuard has expanded its technology platform in a disruptive innovation: the « smart » pedicle screw launched late 2017 and is broadening the scope of applications in dental implantology and surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
SOURCE: https://www.spineguard.com/wp-content/uploads/SpineGuard_PR_Sales-2Q20_EN_vdef-2.pdf
Contacts
SpineGuard
Pierre Jérôme
Chairman & CEO
Tel: +33 1 45 18 45 19
[email protected]
Manuel Lanfossi
CFO
Tel: +33 1 45 18 45 19
[email protected]
Europe / NewCap
Investor Relations & Financial Communication
Mathilde Bohin / Pierre Laurent
Tel: +33 1 44 71 94 94
[email protected]
