PARIS (France), BOULDER (CO, USA), April 17, 2024, – 06:00 pm CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) unique sensing technology using electrical conductivity local measurement in real time to secure and streamline the placement of bone implants, announced today its full-year 2023 financial results, for the financial year ending December 31, 2023, as approved by the Board of Directors on April 17, 2024, and its first quarter 2024 sales.
Pierre Jérôme, co-founder, Chairman and CEO of SpineGuard, said: “Our return to global sales growth in the first quarter of 2024 validates the choice we made to strengthen our US commercial infrastructure and invest in R&D to develop three new products embedding our DSG sensor: the Threaded PediGuard adapted to the correction of scoliosis via anterior approach, the Cannulated PediGuard designed for sacroiliac fusion and the DSG Drill Bit compatible with power drills and surgical navigation in the spine. This significant financial effort to address clearly identified and quickly actionable opportunities, explains the decrease of our net result in 2023. We are building our financial strategy around our unique real time surgical guidance technology, its broad spectrum of applications and its clinical relevance now backed by more than 100,000 surgeries secured and 34 articles published in peer-reviewed scientific journals. Our growth will most likely accelerate in the coming quarters driven by the gradual introduction of these three new products, the impact of our new sales organization as well as our fruitful collaboration with Omnia Medical in the United States and XinRong in China. In parallel, we are in discussions with other potential strategic partners in particular to deploy DSG in dental implantology and surgical robotics.”
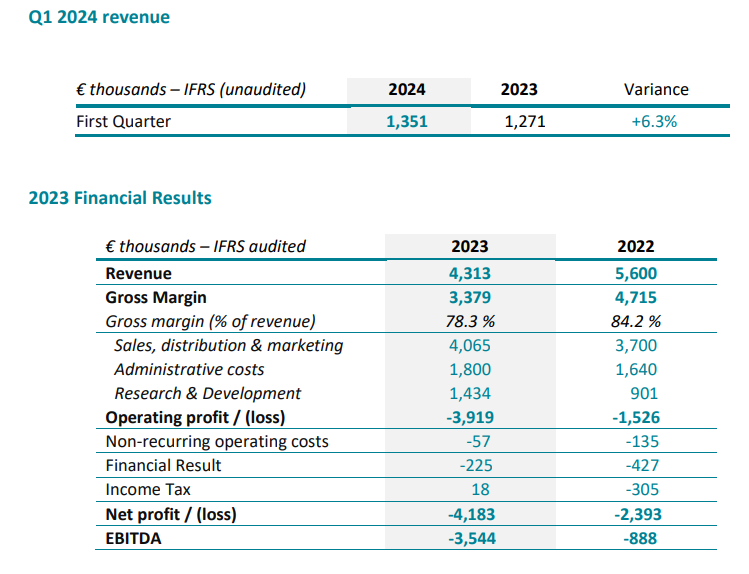
2023 EBITDA impacted by the strengthening of US investment and innovation
As announced in January 2024, 2023 sales amounted to €4,313 K, down -23% at actual exchange rate and -21% cc compared with 2022.
6,138 DSG units were sold in 2023 vs. 5,934 in 2022, representing overall growth of +3%, which confirms the global momentum for DSG technology despite the one-off drop in sales in the United States, with 2,120 units sold in 2023, representing 35% of all units sold.
In the United States, full-year 2023 sales fell by -29% to €2,678 K from €3,785 K in 2022, due to the discontinuation of WishBone Medical’s business, related to financial difficulties of its own, which had exclusive distribution of SpineGuard products in US pediatric orthopedic hospitals. Nevertheless, the new US team put in place by SpineGuard in fiscal 2023 has gradually taken over direct distribution since November. This is reflected in sales, which have grown sequentially over the last three quarters (on the date of publication of this press release).
In the rest of the world, sales rose by +22% for products over the full year, thanks to strong growth in Europe and Latin America, and a major order in China from XinRong Medical Group, SpineGuard’s local partner.
Besides, overall sales were negatively impacted by the discontinuation of royalty income relating to the dental project, following the decision by the Adin Dental Implant Systems Group to halt developments, for its own financial reasons, at the end of the first quarter of 2023.
The gross margin rate for 2023 sets at 78.3%, compared with 84.2% in 2022. This decline is mainly due to the discontinuation of royalty income from the dental project and the decline in US sales, where gross margins are higher than in the rest of the world.
Current operating expenses rose by 16.9%, i.e. €1,058 K, reflecting the financial impact of sales and marketing investments, particularly in the United States, and ongoing R&D innovation.
Non-current expenses amounted to €57 K at December 31, 2023, compared with €135 K at December 31, 2022, and correspond mainly to restructuring costs in the United States.
Operating profit before non-recurring items therefore came to -€3,919 K, at December 31, 2023, compared with -€1,526 K at December 31, 2022.
Net financial expense, at -€225 K, mainly reflects interest payments on debts contracted with Norgine Venture, Harbert European Growth and Bpifrance, and net foreign exchange losses of -€70 K, offsetting income from investments (term accounts) of €45 K, as well as changes in the derivative liability, with no impact on cash, of €213 K.
After taking these items into account, net income would be -€4,183 K in 2023, compared with -€2,393 K in 2022.
Operating working capital stood at €540 K at December 31, 2023, compared with €452 K at December 31, 2022.
Cash and cash equivalents (shown under current financial assets) at December 31, 2023 came to €3,893 K, compared with €4,115 K at December 31, 2022. This change in cash and cash equivalents is mainly due to:
- Cash flow from operations deteriorated to -€3,545 K in 2023 from -€889 K in 2022, and cash used to finance operations increased by €2,247 K to -€3,649 K in 2023, versus -€1,402 K in 2022;
- The change in working capital requirements, which deteriorated by €104 K in 2023, compared with a deterioration of €513 K in 2022, mainly due to the increase in inventories linked to the reduction in US sales, and the increase in prices linked to the unfavorable USD/EURO exchange rate and the impact of the increase in the price of electronic components;
- Partial repayment of principal on bonds taken out with Norgine Venture and Harbert European Growth for €761 K;
- Repayment of principal on the Bpifrance loan in the amount of €49 K;
- Payment of interest on bonds subscribed with Norgine Venture and Harbert European Growth in the amount of €226 K; and
- Equity contributions following drawdowns on the equity financing line (redeemable equity warrants – “BSAR”) for a total gross amount of €3 M and the capital increases carried out in July and December 2023 for a total gross amount of €2 M.
The Company benefits from a repayable advance under COFACE contracts (prospecting insurance) for China. No repayments have been made in respect of the fifth year of amortization of this advance.
Cash position
On March 31, 2024, cash and cash equivalents were €2.5 M.
The Horizon equity line put in place on May 31, 2023 with Nice & Green’s for an amount of €7.5 M, remains undrawn and suspended per the information given at the launch of the capital increase with shareholders’ preferential subscription rights of €1.5 M in December 2023.
Considering these elements as well as its commercial and financial projections, SpineGuard now has cash-flow horizon until 2026.
Significant progress with technology and regulatory
PediGuard Threaded for anterior surgery
The products specifically modified for a precise reading of the drilling depth, and for compatibility with the main instrumentations in the market, are available in the United States and in the process of approval under the new MDR (Medical Device Regulation) Directive in Europe.
Modified Cannulated PediGuard for the sacroiliac joint fusion
Fruit of the collaboration with the US based company Omnia Medical, the design is now completed and the regulatory phase has started in order to obtain the clearance by the FDA (Food and Drug Administration) in the United States.
DSG Universal Drill Bit
The design progressed significantly and SpineGuard is now actively preparing the regulatory phase in the USA. The new range of drill bits embedded with the DSG sensor is intended to be compatible with the main power drills in the orthopedic market, with the dominant surgical navigation system in the US market and with the DSG Connect interface. It is the first step of a plan to rollout commercial “smart” products directly derived from the research programs for robotic application of DSG.
Clearance of the complete range of PediGuard models in China
SpineGuard progresses well with the execution of its plan established in collaboration with its partner XinRong Medical Group and the Franco-Chinese regulatory consultant VVR. It consists in the staged clearance of the PediGuard products that have not yet been approved: Curved PediGuard, “XS” (miniaturized) PediGuard, as well as the Cannulated and Threaded versions.
Smart DSG Pedicle Screw
The collaborative design with Omnia Medical is ongoing. SpineGuard will provide the DSG components that will equip the Screw system of Omnia Medical including the bone breach detector for securing the implantation. This product will target the American market.
MDR Migration towards the new MDR regulation
SpineGuard continues the work with the TÜV SÜD European notified body to obtain its updated certificates in the course of 2024.
Strategic and Technology Development Projects
The SpineGuard R&D team is progressing innovative work. As part of it, the DSG Robotic application advances the bone resection topic, and the use of ultrasound to determine the pedicle drilling entry point. The data collection for Bone Quality Measurement continues, as well as the exploration of the dental application. Along these progresses, SpineGuard systematically protects the new concepts, and as a result the patent portfolio accounts as of today for 14 families and a total of 75 patents and patent applications in numerous countries.
Q1 2024 revenue
SpineGuard’s consolidated sales were up 6% at actual exchange rate (7% cc) in Q1 2024 compared with Q1 2023.
In the United States, first quarter 2024 sales rose by 23% in dollar terms to $1,014 K, vs. $822 K in Q1 2023. This sharp increase is due to the takeover of Wishbone accounts, a significant order from Omnia Medical, and the fruits of the labor of the new U.S. team put in place by SpineGuard last spring.
In the rest of the world, sales rose by +1% for products in the first quarter of 2024. It should be noted that overall growth in Q1 2024 was affected by the absence of royalties (vs. €92 K in Q1 2023) relating to the dental project in collaboration with the Adin company, which was discontinued in Q1 2023. This will be the last quarter to suffer this negative comparative impact.
1,780 DSG units were sold in Q1 2024 vs. 1,621 units in Q1 2023, representing overall growth of +10%.
706 units were sold in the United States, representing 40% of all units sold.
SpineGuard Priorities
SpineGuard continues its sales drive, particularly in the United States, and market rollout of three new products based on DSG technology (the PediGuard Threaded adapted to scoliosis correction via anterior approach, the PediGuard Canulated designed for sacroiliac fusion, and the DSG Universal Drill Bit compatible with power drills and surgical navigation in the spine) in order to return to double-digit growth in 2024.
The Company is also working on clearing the entire PediGuard range in China, as well as establishing strategic partnerships in the dental implantology and surgical robotic fields.
Next events
General Shareholders Meeting on June 5, 2024 (and June 26, 2024, if second summoning)
2024 half-year revenue on July 10, 2024
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices embedding its technology. Over 100,000 surgical procedures have been secured worldwide thanks to DSG® and 34 studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard is expanding the scope of its DSG® technology to the treatment of scoliosis via anterior approach, sacroiliac joint fusion, dental implantology and innovations such as the « smart » pedicle screw and power drill or surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
