Corrigendum: This article has been updated to reflect that MARVEL™ and REFLECT™ are FDA-approved devices, correcting previous descriptions that referred to them as investigational.
Author’s Note:Early-onset scoliosis (EOS) remains a complex condition, and research continues to explore more effective ways of managing it. With this article, my goal was to provide an overview of the different treatment strategies currently available, ranging from non-surgical approaches to advanced surgical systems. For those interested in specific devices, you can also explore the EOS Products link for a comprehensive list. The article turned out longer than I initially expected, but I hope it serves as a useful resource for those of you who are interested in this topic yet may not be fully familiar with the current landscape.
What is Early-Onset Scoliosis (EOS)?
Early-onset scoliosis (EOS), defined as a spinal deformity that appears before the age of 10, is far less common than adolescent idiopathic scoliosis (AIS) but represents one of the most significant challenges in pediatric orthopedics. Its prevalence is estimated at approximately 1 in 5,000–10,000 children (though precise data are limited due to heterogeneous etiologies such as idiopathic, congenital, neuromuscular, and syndromic forms), compared with 1–2% for AIS. Despite its rarity, EOS carries disproportionately high risks: rapid progression during critical growth phases can interfere not only with spinal alignment but also with thoracic development and pulmonary capacity, ultimately affecting survival.
Unlike AIS, EOS cannot be treated primarily with spinal fusion. Early definitive fusion halts spinal and thoracic growth, causing irreversible restriction of pulmonary function. Therefore, the overarching principle in EOS management is “control deformity while preserving growth.” Over the past two decades, this philosophy has driven innovation from serial casting to advanced “smart” implants, transforming what was once a sequence of repeated invasive procedures into an era of motion-preserving and growth-friendly technologies.
This review provides an updated overview of non-surgical and surgical strategies, highlighting FDA-approved options in the United States, investigational systems abroad, and future developments.
What are the Non-Surgical Options?
Before surgical treatment is considered, non-invasive options play an important role in delaying intervention:
- Serial casting (EDF cast): Introduced by Min Mehta, elongation–derotation–flexion casts applied under anesthesia can correct spinal deformity during the first years of life. In some cases of idiopathic EOS diagnosed before age 3, complete resolution is possible. More commonly, casting delays surgery and prevents rapid curve progression.
- Bracing: While highly effective in AIS, bracing has limited value in EOS. It can stabilize moderate curves or provide support between surgeries, but it rarely prevents progression in the long term.
- Physiotherapy and multidisciplinary care: Physical therapy, pulmonary rehabilitation, and coordinated care across specialties (pulmonology, neurology, rehabilitation medicine) help maintain respiratory function and improve quality of life, particularly in neuromuscular EOS.
What are the Growth-Friendly Surgical Systems?
Growing Rods
- Traditional Growing Rods (TGR), FDA-approved, remain a foundation of EOS surgery. Implanted above and below the curve, they allow spinal growth between anchor points and yield about 50–60% correction. Their main drawback is the need for repeated lengthenings under anesthesia every 6–12 months, exposing patients to cumulative surgical and anesthetic risks.
- To address this, MAGEC™ rods—originally developed by NuVasive and now part of Globus Medical (following acquisition)—introduced non-invasive magnetic lengthening controllable in the clinic. FDA-approved, MAGEC™ reduced repeat surgeries by up to 70%. However, device failures occur in ~15% of cases over five years, including rod fracture and magnet malfunction.
- FLYTE™ (AMB Surgical II) represents the next step: a “smart” rod capable of remote adjustment and real-time biomechanical feedback. While promising, it remains investigational, limited to select international trials, and has not yet received FDA approval.
- MARVEL™ (Minimally Invasive Rod for Very Early-Onset Scoliosis), presented in early reports as a minimally invasive distraction system for children under 10. MARVEL received FDA clearance back in 12/2022 and was released for sale/launched mid-2023
- VEPTR, the Vertical Expandable Prosthetic Titanium Rib, designed by Robert Campbell Jr. and FDA-approved, is specifically indicated for children with thoracic insufficiency syndrome. Unlike rods, it primarily expands thoracic volume by separating ribs, permitting growth of the chest wall. It can be lifesaving in this subset but requires repeated expansions and is prone to migration.
Growth-Guided Systems
- Shilla® Growth Guidance System, FDA-approved, enables guided spinal growth without repeated lengthenings. Anchors at curve endpoints allow rods to glide as the child grows. It has shown correction rates of ~60% in idiopathic and neuromuscular EOS but requires significant surgical expertise.
- VerteGlide™ (OrthoPediatrics), FDA-approved in 2023, refines this approach with a smoother sliding mechanism, lowering reoperation rates and showing high promise in early multicenter outcomes.
- The historical Luque Trolley, an early growth-guidance design, is now obsolete due to its high complication rate and lack of regulatory approval.
Vertebral Body Tethering (VBT)
VBT aims to correct scoliosis while preserving motion through growth modulation.
- The Tether™, originally developed by Zimmer Biomet and since 2023 marketed by Highridge Medical, received FDA approval under a Humanitarian Device Exemption (HDE) in 2019. It is indicated for skeletally immature patients (Risser 0–1) with 40°–60° curves. Clinical benefits include motion preservation and faster recovery compared to spinal fusion. However, limitations persist: tether rupture (5–10%), potential overcorrection in very young patients, and eventual conversion to fusion in some cases.
- In Europe, REFLECT™ (Globus Medical) stands as the most advanced next-generation tethering system. With improved biomaterials and intraoperative tension control, early multicenter results suggest fewer mechanical failures and revisions compared to The Tether™. REFLECT was approved under the HDE pathway by the FDA on 5/13/2023.
Dynamic and Motion-Preserving Devices
Beyond tethering, new systems strive for continuous correction without repeated surgeries:
- Nitinol-based systems, which use nickel–titanium alloys with shape-memory and superelastic properties, provide continuous and gentle corrective forces without the need for repeated lengthenings. Their advantages include gradual remodeling of curvature, the ability to accommodate growth stresses, and the potential for fewer surgeries. Evidence comes from animal studies showing significant correction within weeks, as well as early European reports suggesting promising outcomes, although long-term data remain limited.
- Spring Distraction System™ (Cresco Spine): Incorporates internal springs producing gradual, continuous 3D correction. Early European data are promising, reducing repeat interventions while maintaining mobility.
- ApiFix®: A flexible, self-adjusting rod FDA-approved under HDE in 2019 for AIS (manufactured by ApiFix, now part of OrthoPediatrics). While some centers are studying its use in EOS in children ≥6 years, its formal indication remains limited to AIS. Its minimally invasive implantation and motion preservation are attractive, but EOS applications must still be considered experimental.
Which systems are FDA-approved and which are still investigational?
Currently, several systems are FDA-approved for EOS, reflecting two decades of technological evolution:
- Traditional Growing Rods (TGR): Established as a foundation of EOS surgery, providing growth accommodation with scheduled surgical lengthenings.
- MAGEC® Rods (Globus Medical): Magnetic growing rods allowing non-invasive lengthenings in the clinic, substantially reducing repeated surgeries, although long-term mechanical failures remain a concern.
- VEPTR® (Vertical Expandable Prosthetic Titanium Rib): Specifically indicated for thoracic insufficiency syndrome, expanding chest volume and supporting pulmonary development.
- Shilla® Growth Guidance System: Growth-guided rods enabling spinal growth without repeated surgical distractions, particularly effective in idiopathic and neuromuscular EOS.
- VerteGlide™ (OrthoPediatrics): FDA-approved in 2023, refining Shilla® with improved mechanics and fewer reoperations in early outcomes.
- MARVEL™ (Globus Medical): A minimally invasive distraction-based growing rod system for children under 10 with early-onset scoliosis, cleared by the FDA (510(k) K213196). It is designed to help maintain curve correction while allowing continued spinal and thoracic growth. Its key strength lies in providing a growth-friendly solution that reduces the need for frequent invasive surgeries.
- REFLECT™ (Globus Medical): An anterior vertebral body tethering (VBT) system approved by the FDA via Humanitarian Device Exemption (HDE) in May 2023, indicated for skeletally immature patients with progressive idiopathic scoliosis (Cobb angle 30–65°). One of its advantages is offering a motion-preserving alternative to fusion, with the potential for fewer complications compared to earlier tethering systems.
- The Tether® (Highridge Medical): The only vertebral body tethering (VBT) system with FDA approval, under Humanitarian Device Exemption (HDE). Indicated for skeletally immature patients (Risser 0–1) with moderate curves (40°–60°).
- ApiFix® (OrthoPediatrics): Approved under HDE in 2019, but strictly for adolescent idiopathic scoliosis (AIS). Its use in EOS remains off-label and investigational.
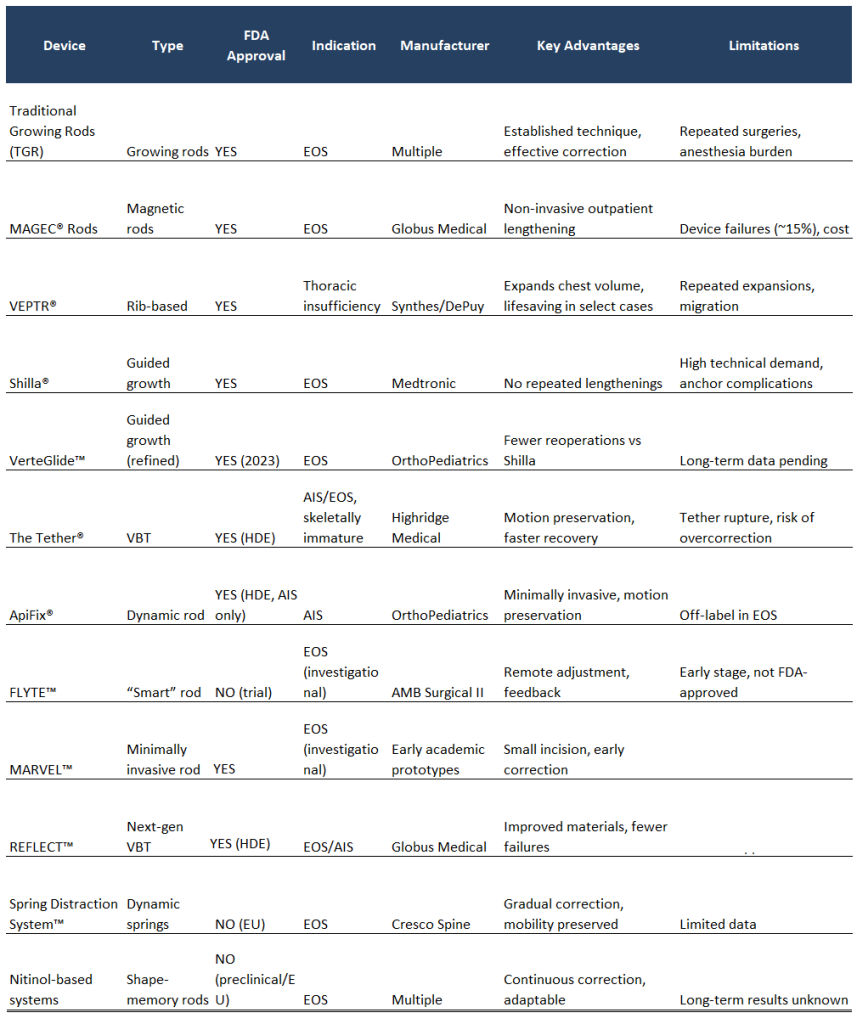
In parallel, multiple devices are under development or in international use but lack FDA approval, restricting their availability in the U.S.:
- FLYTE™ (AMB Surgical II): A “smart” rod with remote control and biomechanical feedback, currently in international trials.
- Nitinol-based systems: Shape-memory rods applying continuous corrective forces; promising in early studies but not yet widely available.
- Spring Distraction System™ (Cresco Spine): Internal spring-based construct providing gradual correction; currently limited to European centers.
- Luque Trolley (historical): An early growth-guidance design, never FDA-approved and now abandoned due to high complication rates.
What Are the Regulatory, Access, and Cost Implications of EOS Devices?
The availability of FDA-approved devices ensures that most EOS patients in the United States are managed with systems that have undergone regulatory evaluation for safety and efficacy. However, investigational systems, particularly in Europe, highlight future directions toward less invasive, dynamic, and smart technologies. The gap between innovation and regulatory approval underscores both the rapid pace of device development and the challenges of ensuring long-term outcomes before widespread adoption.
Beyond the technical challenges, cost remains a critical factor. Systems such as MAGEC® and VBT can easily exceed USD 30,000–50,000 per patient, not including revision surgeries. This restricts their adoption even within hospitals in the United States and Europe, where insurers or public healthcare systems do not always cover the latest-generation devices. As a result, many families continue to face inequities in access, underscoring that innovation must go hand in hand with cost-effectiveness strategies and sustainable financing models.
What Does the Future Hold for EOS Management?
EOS management is entering a transformative phase characterized by:
- Advanced biomaterials: Development of Nitinol alloys with improved fatigue resistance, and exploration of resorbable polymers to reduce the need for implant removal.
- Digital integration: Smart implants with wireless sensors capable of transmitting data on spinal loading and curve progression to clinicians in real time. Combined with AI-driven analytics, this may enable predictive modeling and personalized care.
- Personalized surgery: Advances in 3D printing and surgical planning are enabling patient-specific rods, anchors, and tethers. Early studies suggest improved fit and reduced complication rates.
- Global equity: Access to EOS technologies remains skewed toward high-resource settings. The cost of advanced implants like MAGEC® or VBT is prohibitive in many regions. Addressing global access and cost-effectiveness is an urgent ethical priority.
- Ethical dimensions: Informed consent in pediatrics is evolving. Increasingly, children’s perspectives are considered alongside parental authority, particularly for procedures with long-term quality-of-life implications.
In Our Opinion, Which Are the Most Advanced EOS Devices Based on Current Evidence?
Among FDA-approved systems, MAGEC® rods remain a milestone for non-invasive lengthening, while VerteGlide™, Marvel®, and Reflect® refine guided growth and early scoliosis correction with improved precision and fewer complications. The Tether® preserves spinal motion in selected patients. Among investigational devices, FLYTE™ represents the paradigm of intelligent implants with remote adjustment. These developments mark a transition from static, surgery-dependent constructs to dynamic, growth-preserving systems that may redefine future standards of care.
What Are Our Final Thoughts?
In just two decades, EOS treatment has progressed from repeated invasive surgeries to dynamic, motion-preserving, and increasingly intelligent implants. No single solution fits all patients; best practice relies on tailored device selection, multidisciplinary care, and equal emphasis on radiographic correction and long-term quality of life.
Looking ahead, the trajectory is clear:
- From static implants to dynamic, smart systems with real-time feedback.
- From repeated open surgeries to minimally invasive, outpatient-adjustable technologies.
- From one-size-fits-all to personalized implants designed with digital planning and 3D printing.
The future standard of care will likely combine intelligent growth-preserving devices with AI-driven monitoring, while the central challenge will be ensuring global accessibility and cost-effectiveness. Innovation without equity risks leaving many children behind—making this as much an ethical question as a technological one.
####
Appendix: Emerging Systems Not Included in Main Review
- Braive™ (Medtronic): Under IDE trial for juvenile/adolescent scoliosis. Data pending.
- Nemost® (Euros): CE-marked, mechanically self-expanding rod. Early European use, limited outcomes.
- Kira™: No verifiable clinical data available.
- CurvRITE™: First-in-human trial (2021). Early promising results, no regulatory approval.
- MIScoli™: FDA Breakthrough Device, feasibility trial 2023–2025. Results pending.
References
- Yoon WW, et al. Current concepts in the management of early-onset scoliosis. Spine Deformity. 2021;9(6):1483–1495.
- Smith JT, et al. Long-term outcomes of MAGEC rods in early-onset scoliosis. J Bone Joint Surg Am. 2023;105(12):934–942.
- Thompson R, et al. Comparative effectiveness of Shilla and Vertebral Body Tethering in pediatric scoliosis. Eur Spine J. 2022;31(8):1890–1898.
- Lebel DE, et al. Early multicenter results of REFLECT vertebral body tethering system. Spine J. 2024;24(3):456–463.
- Campbell RM, et al. The VEPTR: Growth-sparing surgical management of thoracic insufficiency. J Bone Joint Surg Am. 2004;86:1659–1674.
- POSNA/SRS EOS Guidelines. Pediatric Orthopaedic Society of North America, 2023.
Compliance Statement
This article is intended exclusively for educational and informational purposes. It is based on peer-reviewed literature, international guidelines, and publicly available regulatory data up to August 2025. It does not replace professional medical advice or individualized recommendations from a pediatric orthopedic specialist. Treatment decisions must always be made in the context of individualized clinical consultation. The author declares no direct financial conflicts of interest with the companies mentioned and has made every effort to present accurate, verified information from academic and regulatory sources.
Resources
- List of EOS Devices: https://thespinemarketgroup.com/category/deformity/early-onset-scoliosis-eos/
- Brochures: https://thespinemarketgroup.com/category/brochures/
- Videos: https://www.youtube.com/channel/UCbwEJb1agj-BvF6k4bJ_M0w
- Pediatric Deformity Video Playlist: https://www.youtube.com/playlist?list=PLM22AATH0vHvCgowFP5WzpmPDmseuuQl-
Cover Image: Globus Medical MAGEC
