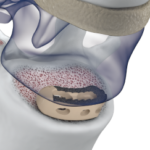
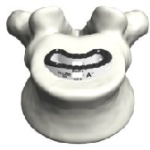
Pivotable Design Prodorth TLIF Cage is intended for single use only and restore the degenerative disc pathologies.Prodorth TLIF Cage is made of PEEK (ASTM F2026) which is a polymer based composite material and Ti6Al4V (ASTM F 136) material. PRODOLPHIN VIDEO ANIMATIONPRODOLPHIN-SGT.Prodorth-Spine.pdf Prodorth TLIF Cages ® provides an uninterrupted guidance during … [Read more...] about PRODOLPHIN™
TLIF
TASMIN®R
Minimal Access – Maximal Effect Posterior lumbar interbody fusion (PLIF) is today the gold standard in the treatment of degenerative disc disease. Most recently, surgical restoration of the sagittal and coronal profile in particular has gained in importance. Whilst physiological curvature of the spine allows for even load distribution, degenerative processes may result in … [Read more...] about TASMIN®R
JULIET®TI TL
JULIET®TI TL is a titanium interbody implant featuring the company’s proprietary Ti-LifeTechnology.Ti-LifeTechnology micro-porous scaffold is designed to mimic the bone trabecular structure, featuring interconnected pores of 600μm to 700μm and an overall porosity of 70-75%—intended to enable cell colonisation and promote bone in-growth.The Juliet Ti PO, OL and TL interbody … [Read more...] about JULIET®TI TL
ADONIS® Exclusive
ADONIS® Exclusive is rewriting standards in the area of thoracolumbar interbody fusion. The titanium coatings of the new ADONIS® Exclusive cages combine the advantages of various materials in one implant. The basis of the implant is a solid Peek core. This core is coated with titanium to increase the surface area and thus also to maximise the contact zone between the implant … [Read more...] about ADONIS® Exclusive
Battalion PC
Battalion PC system aims to provide surgeons with range of implants and instrumentation necessary for achieving optimal lumbar fusions. All implants are titanium-coated and are available in a wide variety of height and footprint options. The implants are titanium-coated for potential osseointegration. Finally, the instrumentation offered in the PC Instrument Sets feature … [Read more...] about Battalion PC
Calix PC
The X-spine System’s Calix PC Spinal Implant System is a generally box or oval-shaped device that has various holes throughout its geometry. Superior and inferior surfaces of the device have titaniumcoated teeth to help prevent implant dislodgement or expulsion once placed in its desired location. The device body is made from polyetheretherketone (PEEK) per ASTM F2026, with … [Read more...] about Calix PC
Camber
The Camber TLIF (Transforaminal Lumbar Interbody Fusion) device is a single-component device manufactured from PEEK-OPTIMA, with one tip and two anterior-posterior titanium-alloy imaging markers. Titanium coating is plasma sprayed onto the device integration surfaces. The Camber is anatomically shaped to fit the lumbar disc space optimally, with two different lengths and … [Read more...] about Camber
EOS
EOS is Aurora Spine's modern, minimally invasive TLIF interbody fusion system. Manufactured out of PEEK, each cage is precision coated with TiNano®, Aurora Spine's titanium plasma spray coating. Features: Aurora Spine cages feature a self-distracting nose, as well as teeth on the inferior and superior surfaces to help prevent retropulsion and migration. Graft windows … [Read more...] about EOS
FortiCore TLIF
FortiCore TLIF combines the benefits of PEEK and titanium creating the most scientifically advanced interbody fusion devices. FortiCore interbody fusion devices are comprised of a PEEK center with a deeply porous titanium scaffold. PEEK Optima® by Invibio is also injection molded into the scaffold for exceptional integration. This unique combination of technologies is designed … [Read more...] about FortiCore TLIF
FORZA PTC Spacer System
FORZA PTC Spacer System has been designed to help optimize Transforaminal Lumbar Interbody Fusion (TLIF), procedure with surgeon designed implants and instruments. FORZA PTC Spacers offer a unique technology that combines PEEK and titanium into a porous interbody solution for the lumbar spine. This PEEK/Titanium hybrid is designed with a 3D porous endplate that allows the … [Read more...] about FORZA PTC Spacer System
Lucent Ti-Bond
Lucent® Ti-Bond™ is a line of PEEK interbody implants with a plasma-sprayed titanium porous coating. The company’s Ti-Bond coating consists of random, unconnected titanium pores, which are biomechanically adhered through a plasma vacuum spray process to the superior and inferior surfaces of its PEEK interbody implants. This results in an ideal bone-opposing surface while … [Read more...] about Lucent Ti-Bond
Tyber Medical TLIF
The comprehensive and versatile Tyber Medical TLIF Interbody Spacer was designed for ease of use. Benefits: Optional TyPEEK™ Titanium Plasma Spray:TyPEEK provides an outstanding, new solution for fusion procedures by combining the properties of a Titanium plasma spray with the modulus of PEEK. Anatomical Endplate Contact Large Graft Window PEEK-Optima™ Core … [Read more...] about Tyber Medical TLIF