Tim Denning (medium.com)–Knowing when not to talk is an art. As a corporate man by day, and an entrepreneur by night, I’ve attended my fair share of meetings over the last decade or so. Meetings can be an odd experience. Before you know it the meeting can get out of control. Leaders with pinstripe suits […]
NEWS
The “Poverty Spirit” Device Rep
Kevin Brown–Since I spend a lot of time in my car I always try to find vehicles that are comfortable and have some semblance of year-end resale after 40k miles. On one particular day I had just traded in for a new truck and drove up to a gaggle of reps in the hospital parking […]
Surgalign Holdings, Inc. Announces Completion of Acquisition of Holo Surgical Inc. and its ARAI Digital Surgery Platform
DEERFIELD, Ill., Oct. 23, 2020 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a global pure-play spine company focused on advancing spine surgery including through the application of digital technologies to improve patient outcomes, today announces the completion of the acquisition of Holo Surgical Inc., (‘Holosurgical’), a Chicago-based private technology company developing the revolutionary Augmented […]
Spine Companies worth Knowing… (Part 1)
In today’s spine market, there are more than 400 competing companies. Some of them are well known for being market leaders and others have gradually gained greater relevance. However, there are still many companies that have interesting products that are quite unknown to people. Today we want to present four companies that, either for having […]
Seaspine Receives CE Mark Approval for Daytona® Small Stature posterior fixation system
SeaSpine® Daytona® Small Stature posterior fixation system is now CE Marked! This system is designed to address standard to complex deformity cases in smaller-sized patients who need a lower profile construct due to anatomy constraints. Daytona® Small Stature VIDEO ANIMATION Daytona® Small Stature Spinal System The Daytona® Small Stature Spinal System is designed to address standard to […]
SpineGuard strengthens its intellectual property with a new patent granted in China
Paris (France) and Boulder (CO, USA), October 20, 2020 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the grant of a new patent in China, after its grant in Russia and Singapore as announced last April. This […]
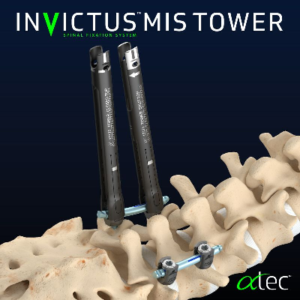
ATEC Extends InVictus MIS Platform With Commercial Release of InVictus MIS Tower
CARLSBAD, Calif., Oct. 19, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today the commercial release of the InVictus™ MIS Tower, an elegant and intuitive system designed for minimally invasive percutaneous fixation. InVictus MIS Tower is the next evolution of InVictus™, […]
Implanet and SeaSpine announce FDA clearance of the Mariner Cap System
Bordeaux, Boston, October 19, 2020 – 5.45 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, and SeaSpine Holdings Corporation (NASDAQ: SPNE) today announce the clearance of the Mariner Cap System in the United States, which combines SeaSpine’s Mariner® pedicle screw system with […]
SpineGuard reports third-quarter 2020 revenue
Paris (France) and Boulder (CO, USA), October 15, 2020 – 18:00 CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today that its third-quarter revenue was €1.3 million. Pierre JEROME, Chairman & CEO of SpineGuard, said: […]
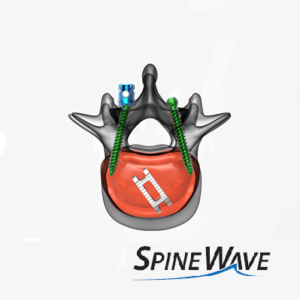
Spine Wave Announces the Commercial Launch of the Salvo® 4.75 mm Spine System
SHELTON, Conn., Oct. 15, 2020 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the successful completion of its limited market release and the launch of the Salvo® 4.75 mm Spine System, and with it, the company’s expansion into the market for thoracolumbar spinal fixation systems featuring modular screw design. The Salvo® 4.75 mm Spine System will […]
NuVasive Announces New Organizational Structure to Align Global Commercial Operations and Product & Services Organization with Long-Term Strategy
SAN DIEGO, Oct. 14, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced a new organizational and leadership structure for its global commercial operations as well as its product and services organization, which were previously consolidated under the role of the Company’s […]
BAGUERA® C in the US – Investigational Device Exemption (IDE) FDA Approval 2 Levels
Following the recent FDA approval to initiate a pivotal IDE clinical study for the BAGUERA® C artificial cervical disc at 1-level, Spineart is proud to announce that it has received approval from the FDA to initiate as well a pivotal IDE clinical study for BAGUERA® C at two contiguous levels.Both studies will be randomized controlled trials comparing the […]
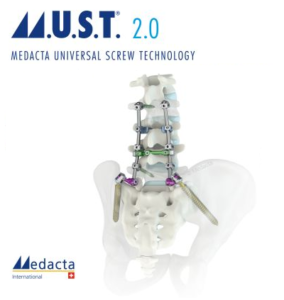
Medacta Launches M.U.S.T. 2.0 Instrumentation to Expand its Offerings for Spine Pathologies
CASTEL SAN PIETRO, 13 October 2020 – Medacta is proud to announce the release of the M.U.S.T. 2.0 instrumentation, a next generation solution for spine surgery designed to simplify the procedure and provide robust instruments in open surgery when treating degenerative spine pathologies. The M.U.S.T. 2.0 system is the next generation of M.U.S.T. instruments that incorporates an […]
Genesys Spine Announces First 3D Printed Non-Screw Based Cervical Stand-Alone Cage
AUSTIN, Texas, Oct. 13, 2020 /PRNewswire/ — Genesys Spine is pleased to announce the launch of the first 3D Printed Non-Screw Based Cervical Stand-Alone Cage in the United States. The AIS-C 3DP Stand-Alone Cervical Cage consists of a 3D printed cervical interbody cage combined with titanium anchors enabling bony fusion and fixation with direct anterior access and a […]
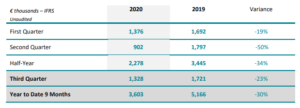
Implanet Reports Revenue of €1.75 Million For Q3 2020, Marking the Resumption of Surgeries in France and Abroad
IMPLANET (Paris:ALIMP), (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, today announces its revenue for the third quarter and first nine months to September 30, 2020. Ludovic Lastennet, CEO of Implanet, commented: “In keeping with the signs observed before the summer, the third […]
First Clinical Cases in the USA to treat vertebral fracture with Spinal Implant Product V-STRUT© developed by HYPREVENTION
PESSAC, France, Oct. 12, 2020 /PRNewswire/ — Hyprevention team is pleased to announce the first clinical cases performed in the USA with the V-STRUT© Vertebral Implant product to treat vertebral fractures. The FDA cleared V-STRUT© Vertebral Implant is indicated for use in the treatment of vertebral fractures due to osteoporosis or bone metastasis. Two elderly patients presenting with osteoporosis and severe […]
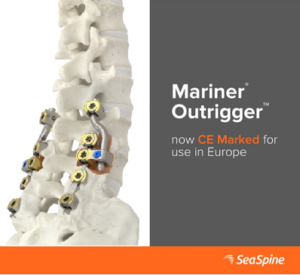
Seaspine Receives CE Mark Approval for Mariner® Outrigger™ posterior fixation system
SeaSpine® Mariner® Outrigger™ posterior fixation system is now CE Marked! This system offers unique clinical solutions by providing diversified implants to enable complex revision and extension spine procedures. Mariner® Outrigger™ Video Animation The Mariner® Outrigger™ Posterior Fixation System features thoughtfully designed, purposeful instrumentation to enhance surgical flow. The system offers unique clinical solutions by providing diversified implants to enable […]
CTL Amedica Secures US Patent for Articulating Implant Insertion System
DALLAS (PRWEB) OCTOBER 08, 2020–The United States Patent and Trademark Office has granted CTL Amedica Corporation patent number 10,765,529 for articulating intervertebral devices, related tools, systems and methods. CTL Amedica’s newly patented system solves many complex challenges that are associated with traditional transforaminal lumbar interbody fusion (TLIF), an operation performed on the lower back to remove […]
SMAIO announces the FDA 510k approval of the KHEIRON® spinal fixation system
SMAIO is delighted to share that our spinal fixation system KHEIRON® just received FDA-510(k) clearance.This big step for SMAIO will allow us to start commercializing our innovative solutions on the US market and will sustain our international development. KHEIRON is a Posterior thoraco-lumbar fixation system offering a full range of implants to adress the main […]
MEDICREA Reports Third Quarter 2020 Sales
Lyon and New York, October 8, 2020 – The MEDICREA® Group (Euronext Growth Paris: FR0004178572 – ALMED ; OTCQX Best Market –MRNTF), pioneering the transformation of spinal surgery through Artificial Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, publishes sales for the third […]
10 Virtual booths to visit before NASS 2020 ends!
NASS 2020 is about to end! Yesterday we talked about market trends and those products that the market leaders had highlighted. Today, before the end of this Congress, we wanted to point out some Virtual Booths that we find interesting for their content or for the products they present: 1.- Centinel Spine: Centinel Spine is […]
NeuroStructures announces the FDA 510k approval of the Oculus™-SA Lumbar Cage System
NeuroStructures is excited and pleased to announce the FDA 510k approval of the Oculus™-SA Lumbar Cage System, which features our “Neuro-Lock” surface technology to enhance fusion. The Oculus™-SA allows the surgeon to use multiple integrated screw construct options for any surgeons’ preference. Along with a zero profile design and locking plate for secure visual confirmation, […]
What are the main products that market leaders are exhibiting at NASS 2020?
We are already at NASS 2020! Although with the limitations of being virtual, a large part of the Spine Companies have maintained their participation in this year complicated by COVID. After visiting the different virtual booths we want to share with you our impressions. We consider that every year the NASS 2020 is an indicator […]
Spinal Elements Announces Launch of Initial Public Offering
CARLSBAD, Calif., Oct. 08, 2020 (GLOBE NEWSWIRE) — Spinal Elements Holdings, Inc. (“Spinal Elements”), a CA-based spine technology company, today announced the launch of its initial public offering of 7,700,000 shares of its common stock at an anticipated initial public offering price between $13.00 and $15.00 per share pursuant to a registration statement filed by […]
ATEC Announces Preliminary Third Quarter 2020 Financial Results
CARLSBAD, Calif., Oct. 07, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today preliminary, unaudited financial results for the third quarter ended September 30, 2020. Preliminary, Unaudited Third Quarter 2020 Financial Results Preliminary, unaudited third quarter 2020 results are expected to reflect U.S. revenue growth of 41% […]
Stryker announced the full launch of the Niagara Lateral Access System
Stryker unveils their latest addition to its comprehensive minimally invasive portfolio for lateral spine surgery. Today, Stryker announced the full launch of the Niagara Lateral Access System following the completion of 600 surgeries.The company will showcase its lateral portfolio during the virtual North American Spine Society (NASS) Annual Meeting, Oct. 6-9, 2020. Niagara Lateral Access […]
ATEC Announces Commercial Release of Distinct, Comprehensive TLIF Approach
CARLSBAD, Calif., Oct. 06, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today the commercial releases of two new solutions designed to elevate the ATEC transforaminal lumbar interbody fusion (TLIF) clinical experience. The Sigma Access System and the […]