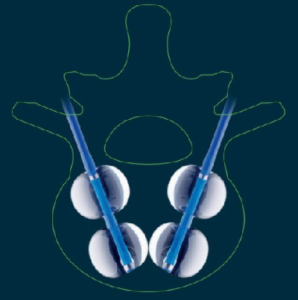
ALTSTAETTEN, Switzerland, Nov. 10, 2020 /PRNewswire/ — icotec ag, the leading medical device manufacture of Carbon/PEEK spinal implants, announces the U.S. Food and Drug Administration (FDA) 510(k) clearance to market the VADER® Pedicle – System for use with bone cement for augmentation or without. The VADER® Pedicle System is intended to restore the integrity of the spinal column even […]
NEWS
Dymicron™ appoints Armen Khachatryan, M.D. to Board of Directors English
OREM, Utah, Nov. 10, 2020 /PRNewswire/ — Dymicron, a privately held, innovative medical device company developing a next generation cervical artificial disc, Triadyme-C™, with a proprietary polycrystalline diamond material and novel, Tri-Lobe articulating design, today announced that Armen Khachatryan, M.D. has been appointed to the Company’s Board of Directors. “We are delighted to welcome Dr. Khachatryan to Dymicron’s Board,” […]
SeaSpine Reports Third Quarter 2020 Financial Results
CARLSBAD, Calif., Nov. 09, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced financial results for the three-months ended September 30, 2020. Summary of Third Quarter 2020 Financial Results and Recent Accomplishments Revenue of $43.2 million, an increase […]
NuVasive’s Thoracolumbar Interbody Portfolio Receives First Clearance in the US for the Treatment of Sagittal Deformities
SAN DIEGO, Nov. 4, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its thoracolumbar interbody portfolio to include the treatment of multi-level sagittal deformities of the […]
Stryker elects Giovanni Caforio to Board of Directors
Kalamazoo, Michigan, Nov. 09, 2020 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) announced today that Giovanni Caforio, M.D., Chairman and Chief Executive Officer of Bristol Myers Squibb, has been elected to its Board of Directors, effective December 1, 2020. “Giovanni adds extensive experience to our board as both a physician and business leader,” said Kevin Lobo, Chairman and […]
Medacta USA and OREF Launch 2020 Medacta Challenge Giving Campaign
ROSEMONT, ILL. AND FRANKLIN, TENN. (PRWEB) NOVEMBER 09, 2020–Medacta USA, a subsidiary of the Medacta Group, specializing in the design and production of innovative joint replacement and spine surgery products, and the Orthopaedic Research and Education Foundation (OREF), today announced the launch of the 2020 Medacta Challenge campaign. The program, now in its fifth full year, […]
Astura Medical Receives FDA 510(k) Clearance For Dolomite Stand-Alone Anterior Cervical Stabilization System
IRVING, TX – November 9, 2020 – Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Dolomite Stand-Alone Anterior Cervical Stabilization System. Expanding on the rapid adoption and recent success of the company’s other integrated plate and spacer technologies […]
Zimmer Biomet Announces Third Quarter 2020 Financial Results
WARSAW, Ind., Nov. 6, 2020 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today reported financial results for the quarter ended September 30, 2020. The Company reported third quarter net sales of $1.929 billion, an increase of 2.0% over the prior year period, and an increase of 1.1% on a constant currency basis. Net earnings for the third […]
ATEC Reports Third Quarter 2020 Financial Results and Recent Corporate Highlights
CARLSBAD, Calif., Nov. 05, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a provider of innovative spine surgery solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended September 30, 2020, and recent corporate highlights. Third Quarter 2020 Financial Results Total revenue of $41.2 million, including U.S. […]
Safe Orthopaedics starts construction of its Innovation and Industrial Production Center in Fleurieux-sur-l’Arbresle
Éragny-sur-Oise, France, November 5th, 2020, 17h35 CET – Safe Orthopaedics (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces the construction of its Innovation and Industrial Production Center and of a new integrated production Unit in Fleurieux-sur-l’Arbresle (Rhône, France). […]
Implanet signs distribution agreement for its JAZZ® platform products in Mexico
Bordeaux, Boston, November 4, 2020 – 5.45 pm CET: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, today announces the regulatory approval by Cofepris[1] of its products based on the JAZZ® Spine product range and the signing of a distribution contract with Novovascular Technologies for […]
Stop’n GO Double Balloon Catheter (Quattroplasty®) Provides a New Standard in Minimally Invasive Treatment of Vertebral Fractures
BOSTON, Nov. 3, 2020 /PRNewswire/ — Joline Medical LLC and Joline GmbH & CO. KG announce that the companies have performed the first United States case utilizing Joline’s Stop’n GO Double Balloon Catheter (Quattroplasty®) products to treat vertebral compression fractures. The initial case was performed by Dr. John Morrison, Neurosurgeon of Delray Beach, FL. The Stop’n GO Double Balloon Catheter (Quattroplasty®) and accompanying specialized instrumentation are FDA […]
SeaSpine Announces Publication of Results from a Cellular Bone Graft Study in The Journal of Bone and Joint Surgery (JBJS)
CARLSBAD, Calif., Nov. 03, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the publication of results from a preclinical study, “Examination of the Role of Cells in Commercially Available Cellular Allografts in Spine Fusion: An in vivo animal study,” […]
Aurora Spine Announces Commercial Launch of The SOLO™ System
CARLSBAD, Calif., Nov. 02, 2020 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has commercially launched its proprietary SOLOTM ALIF Stand-Alone Cage system to its innovative suite of MIS products used in spinal surgery. As […]
Centinel Spine prodisc® C Patient-Ambassador Brian Gay Wins 5th PGA Title
WEST CHESTER, Pa., Nov. 2, 2020 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today congratulated Centinel Spine patient-spokesperson Brian Gay for winning the PGA Bermuda Championship. The victory marks Gay’s 5th PGA TOUR win, and his first win in nearly eight years—becoming the oldest TOUR winner since 2015. Centinel Spine began working in […]
INDIUS Medical Technologies is proud to announce the US FDA 510K clearance of the ACURA Thoracolumbar Posterior Stabilization System
Indius Medical Technologies, an Indo – US multinational Startup focused on the development of US FDA compliant global quality products for the treatment of spinal disorders has FDA clearance for the ACURA Thoracolumbar Posterior System. The ACURA System features the following implants: Paediatric Screw Head Profile with Adult Load Bearing Strength enables O.R. Efficiency Anti-Splay […]
Spinal Elements® Announces the Expansion of its MIS Ultra™ Offering with the Full Market Release of the Lucent® XP Wide Expandable Device for MIS TLIF
Carlsbad, CA – October 30, 2020 – Spinal Elements, a spine technology company, today announced the newest introduction in its MIS Ultra™ suite of products with the full release of the Lucent® XP Wide expandable interbody device. This addition to Spinal Elements’ expandableinterbody portfolio offers surgeons a wider footprint option than previously available and is […]
Senior Leadership Appointments at TrackX Technology, Inc. : Ray Oktavec & Chris Helps
CHAPEL HILL, N.C., Oct. 30, 2020 /PRNewswire/ — TrackX Technology, Inc., a Chapel Hill, NC based medical technology leader in virtual live fluoroscopy and instrument tracking, announced today two senior leadership appointments that will support TrackX’s nationwide expansion and continued growth. Ray Oktavec joins TrackX as Vice President of Sales with responsibilities for building and growing the commercial sales model and rapidly […]
Simplify Medical Completes PMA Submission for 2-Level Simplify Disc IDE Study
SUNNYVALE, Calif., Oct. 29, 2020 (GLOBE NEWSWIRE) — Simplify Medical, Inc., maker of the Simplify® Cervical Artificial Disc, announced today the completion of the Pre-Market Approval (PMA) submission for the 2-level Simplify Disc IDE Study to the U.S. Food and Drug Administration (FDA). The Simplify Disc is designed for MRI compatibility, physiologic motion, and anatomical height-matching, […]
Is the market for Minimally Invasive Screws growing? Updated list of 100+ competing products!
Growth is expected in the MIS spinal implant markets as a result of the physiological and surgical advantages of MIS procedures and mailnly due to the use of the Robotic technologies than make the procedure easier safer and less invasive. According to ResearchAndMarkets.com, the U.S. minimally invasive spinal implants market was valued at $1.8 billion […]
Stryker reports third quarter 2020 operating results
Kalamazoo, Michigan, Oct. 29, 2020 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) reported operating results for the third quarter of 2020: The response to the COVID-19 pandemic has included unprecedented measures to slow the spread of the virus taken by local governments and health care authorities globally, including the postponement of deferrable medical procedures and social contact […]
Xtant Medical Announces Third Quarter 2020 Financial Results
BELGRADE, Mont., Oct. 29, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the third quarter ended September 30, 2020. Third Quarter 2020 Financial Highlights: Revenue for the third quarter of […]
NuVasive Announces Third Quarter 2020 Financial Results
SAN DIEGO, Oct. 29, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced financial results for the quarter ended September 30, 2020. Third Quarter 2020 Highlights Net sales increased 1.5% to $295.3 million, or 1.2% on a constant currency basis; GAAP operating margin […]
Nvision Biomedical Technologies: First FDA clearance for an osteotomy wedge system made of PEEK-OPTIMA™ HA Enhanced
San Antonio, Texas/ Thornton Cleveleys (UK), October 28, 2020 – Nvision Biomedical Technologies, a San Antonio-based medical device and implant manufacturer, has FDA clearance for the first osteotomy wedge system made from PEEK-OPTIMA HA Enhanced, a polymer from Invibio Biomaterial Solutions (‘Invibio’). The Trigon® Stand-Alone Osteotomy Wedge Fixation System has osteoconductive properties that promote multi-directional bone healing and […]
Nexxt Spine Marks 11 Year Anniversary
October 28, 2020- Noblesville, IN- Nexxt Spine, LLC a leading manufacturer of spinal solutions celebrates 11 years in business. October 20, 2009 marks the date the first Nexxt Spine patient underwent surgery in Tennessee. Fast forward 11 years and the company has grown into a respected name in the field; owning a portfolio that rivels […]
Globus Medical Reports Third Quarter 2020 Results
AUDUBON, Pa., Oct. 28, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the quarter ended September 30, 2020. Worldwide net sales were $216.1 million, an increase of 10.1% as compared to the third quarter of 2019 GAAP net income was $44.2 million, an increase of 15.4% […]
Centinel Spine Launches New Program to Expand Reimbursement Coverage for prodisc® L Lumbar Total Disc Replacement
WEST CHESTER, Pa., Oct. 27, 2020 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced the launch of the Patient Access Program, a program designed to further expand patient access to the prodisc® L Lumbar Total Disc Replacement (TDR) system. The Patient Access Program will be particularly focused on providing reimbursement support […]