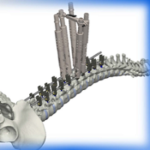
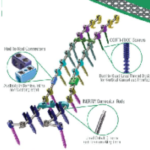
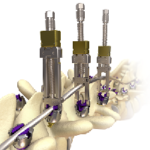
The Canaveral Deformity System is designed to treat advanced complex deformity conditions. Canaveral Deformity FloSpine Brochure.pdf Features: Fast Set up Excellent Correction Adapt easily to a variety of curves Elite Locking screw drivers Screw Inserter and Headbody delivery instruments for in situ assembly Matched color coded taps to screw diameters for fast … [Read more...] about Canaveral Deformity System
NEWS
CD horizon® Legacy™ Spinal System
The CD Horizon® Legacy™ Spinal System can be used to treat scoliosis, a condition in which the spine develops one or more abnormal, side-to-side curves that in turn may affect the body’s overall balance and alignment, as well as possibly lead to other physical and health problems.The CD Horizon Legacy Spinal System is one of the most popular systems for the surgical treatment … [Read more...] about CD horizon® Legacy™ Spinal System
CREO® thoracolumbar stabilization system
The CREO® thoracolumbar stabilization system enhances efficiency and ease of use with intuitively designed instruments and a complete array of implant options for treating complex spinal pathologies with one system. Introducing the lowest profile 4.75 stabilization system on the market, CREO® 4.75 provides surgical solutions for small stature and pediatric patients. CREO® 5.5mm … [Read more...] about CREO® thoracolumbar stabilization system
DENALI® Deformity Spinal System
The DENALI® Deformity Spinal System is a top-loading spinal system featuring off-axis screw height adjustment and offering a complete array of screws, rod connectors, and hooks, coupled with easy-to-use instrumentation. This comprehensive system is poised to address a wide range of complex spinal pathologies. Features Complete Offering of Polyaxial Screws, Monoaxial … [Read more...] about DENALI® Deformity Spinal System
Daytona® System Seaspine
The Daytona® System utilizes the technology of our Malibu™ pedicle screw system to address deformity cases ranging from standard to complex. Unique implant designs, advanced materials and innovative instrumentation creates a highly flexible and intuitive system. Daytona Deformity-Seaspine.pdf Features: Extended tabs for 30mm of reduction Integrated locking cap for … [Read more...] about Daytona® System Seaspine
EVEREST® Deformity Spinal System
The EVEREST® Deformity Spinal System features a top-loading pedicle screw in a variety of screw types and the ability to accommodate titanium and cobalt chrome rods of two different diameters. The revolutionary instrumentation is designed to address the most difficult correction maneuvers for complex spinal pathologies. EVEREST VIDEO ANIMATION EVEREST Minimally … [Read more...] about EVEREST® Deformity Spinal System
Excella III-D® Spinal Deformity System
The Innovasis Excella III-D system is the perfect combination of practicality and efficiency. Through ingenuitive instrumentation that isn't overly complicated, we make complicating surgery easier. The Excella®-M Spinal System consists of 6AL-4V Titanium alloy implants meant to be used in a system. The monoaxial bone screws are offered in a variety of different lengths ranging … [Read more...] about Excella III-D® Spinal Deformity System
EXPEDIUM Spine System
The EXPEDIUM Spine System is a innovative spine solution with technological advancements that truly differentiate it from other systems available. The EXPEDIUM Spine System is a rod based platform. The system consists of the following: • Polyaxial Screws (Single Innie, Dual Innie, Favored Angle Screw) • Monoaxial Screws • Extended Tab Implants • Hooks • Cable and Wires • … [Read more...] about EXPEDIUM Spine System
Firebird Deformity
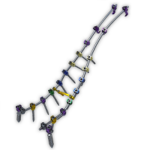
The Firebird Deformity Correction System offers even greater options for patients with spinal deformity. When surgically treating a variety of thoracolumbar and sacral pathologies, the Firebird Deformity Correction System offers additional implant and instrument options needed to perform complex spine procedures. Firebird VIDEO ANIMATION Firebird.Brochure-Orthofix.pdf … [Read more...] about Firebird Deformity
ILIAD Spinal Fixation System
ILIAD Spinal Fixation System provides simple and comprehensive stabilization solutions for spinal fixation. The Patented Reverse DOVETAIL Locking System with LINEAR SLOT is a foundation of the ILIADTM system. This unique thread design practically eliminates cross threading, prevents splaying of the screw head and increase the holding moment up to 30Nm. ILIAD Spine System … [Read more...] about ILIAD Spinal Fixation System
INERTIA Deformity Correxxion System
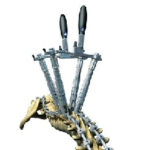
The INERTIA® Deformity Correxxion System, an extension to the Inertia Pedicle Screw System, was designed to address a greater range of complex spinal pathologies from T1 to the Pelvis. INERTIA Deformity Correxxion System VIDEO ANIMATION Inertia Deformity.STG-Nexxt Spine.pdf Inertia Deformity Correxxion System provides the addition of Colbolt Chrome Rods, Uniplanar and … [Read more...] about INERTIA Deformity Correxxion System
Invictus™
Invictus is ATEC’s comprehensive spinal fixation platform, designed to treat a range of pathologies with intraoperative adaptability and surgical predictability through an open, MIS, or hybrid approach. Key Features DEPENDABLE: Leverages patented and dependable helical flange tulip technology to reduce the potential to cross-thread and help eliminate tulip … [Read more...] about Invictus™
LANCER™
The ChoiceSpine LANCER™ Open Pedicle Screw System is a posterior spinal fixation system consisting of various polyaxial screws, rods, cross-connectors, and hooks to accommodate various spinal anatomies. LANCER™ is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion for degenerative disc disease, … [Read more...] about LANCER™
Mercury Total Spine
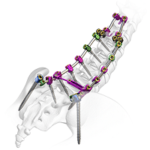
Mercury is the Deformity and Iliac fixation system from Spinal Elements. Mercury System VIDEO ANIMATIONMercury Total Spine.Brochure-Spinal Elements.pdf Features: Multiple screw head optionsScrew sizes to match any patient anatomyVarious rod connectors for surgical versatility About Spinal Deformity Scoliosis is an abnormal curvature of the spine that can … [Read more...] about Mercury Total Spine
MESA® 2 Deformity Spinal System
The next-generation Mesa 2 screws are top-loading, low-profile, and feature Zero-Torque Technology . The streamlined instrumentation is designed for efficiency and ease of use. This system is poised to address the most difficult correction maneuvers for complex spinal pathologies. MESA 2 Deformity VIDEO ANIMATION Features: Dual-lead Thread for Screw Faster Insertion … [Read more...] about MESA® 2 Deformity Spinal System
mont blanc 3D+
The mont blanc 3D+ is a system of implants and instruments for the correction of idiopathic scoliosis by posterior approach.Its is an Implant system for vertebral column inner fixation at thoracolumbar level by posterior approach open surgery, devised for global direct correction of severe spine deformities. mont blanc 3D+ VIDEO ANIMATION mont blanc … [Read more...] about mont blanc 3D+
MUST VBD
The MUST VBD Set has been developed to address some of the challenges of the derotation manoeuvres.The MUST family of products includes a wide selection of deformity specific implants and instruments to support the Medacta Spine philosophy of patient driven, pathology specific solutions.The Medacta Unconstrained Screw Technology [M.U.S.T.] pedicle screw system has been designed … [Read more...] about MUST VBD
MESA® Deformity Spinal System
Top-loading, low-profile spinal system featuring Zero-Torque Technology and offering a variety of screw types, coupled with revolutionary instrumentation and poised to address the most difficult correction maneuvers for complex spinal pathologies. MESA Deformity VIDEO ANIMATION Mesa-SGT.Stryker (K2M).pdf Features: Zero-Torque Technology® No Profile Above the Rod … [Read more...] about MESA® Deformity Spinal System
PIVOT
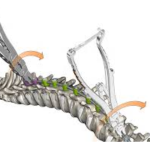
PIVOT is Spinal Deformity System with 3D Derotation. It is designed specifically for derotation in correcting deformity:Reliable corrective performance and controllable corrective operation and Innovative and clinically verified technique. Pivot-Brochure-Kanchui.pdf Features and Benefits: Triangular structure holding up vertebrae integration and derotation accomplished … [Read more...] about PIVOT