Today I read a comment on LinkedIn by Mace Horoff about loyalty in medical devices. It is a message that is more than applicable to the spine implants world, where strong and long-lasting relationships with customers are built over many years. In this business, we don’t just manage accounts. We build real human relationships. We get to know families, share trips, attend … [Read more...] about Loyalty in the Spine Industry: Real, Valuable… and Never Permanent
NEWS
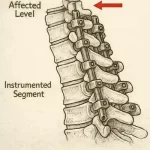
Proximal Junctional Kyphosis (PJK): A Common Issue After Long Spinal Fusions
Proximal Junctional Kyphosis (PJK) is a postoperative spinal deformity defined by an abnormal increase in kyphosis at the upper end of a spinal fusion construct. In simple terms, it occurs at the transition between a rigid, instrumented segment of the spine and the adjacent, non-instrumented vertebrae above. This junction is biomechanically vulnerable. When a long and rigid … [Read more...] about Proximal Junctional Kyphosis (PJK): A Common Issue After Long Spinal Fusions
Become Our Sponsor: It’s Not Advertising. It’s Industry Visibility and Positioning
Increase your business opportunities within the global spine industry. We connect companies with decision-makers, surgeons, distributors, and industry professionals worldwide through independent news, market insights, and clinical content. By becoming a sponsor, your company benefits from: • Increased brand visibility within a highly specialized global spine audience• … [Read more...] about Become Our Sponsor: It’s Not Advertising. It’s Industry Visibility and Positioning
We are proud to announce that Rudischhauser Surgical Instruments and Implants Manufacturing will once again be a sponsor of SPINEMarketGroup in 2026!
Thank you, Rudischhauser Surgical Instruments and Implants Manufacturing! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About Rudischhauser Surgical Instruments and Implants Manufacturing Your partner and OE-Manufacturer of … [Read more...] about We are proud to announce that Rudischhauser Surgical Instruments and Implants Manufacturing will once again be a sponsor of SPINEMarketGroup in 2026!
Nivalon Medical Produces the World’s First Fully Patient-Specific, Motion-Preserving, Metal-Free Spinal Implant Using AI and Advanced Ceramic 3D Printing
YOUNGSTOWN, Ohio, Jan. 29, 2026 /PRNewswire/ -- Nivalon Medical Technologies Inc. has successfully produced the world's first fully patient-specific, motion-preserving spinal implant built entirely without metal, using AI-driven design and advanced ceramic 3D printing. The breakthrough device combines a proprietary zirconia-toughened alumina (ZTA) ceramic … [Read more...] about Nivalon Medical Produces the World’s First Fully Patient-Specific, Motion-Preserving, Metal-Free Spinal Implant Using AI and Advanced Ceramic 3D Printing
South Korea Spine Implant Market: Companies, Trends, Growth. How Clinical excellence, Cost discipline, and Global ambition come together
South Korea is not the largest spine implant market in the world, but it is one of the most strategically interesting. It combines three factors that rarely converge with such intensity: highly technical surgeons, very specialized hospitals, and constant price pressure. The result is an ecosystem where innovation is developed rapidly, validated efficiently, and industrialized … [Read more...] about South Korea Spine Implant Market: Companies, Trends, Growth. How Clinical excellence, Cost discipline, and Global ambition come together
Cytogen and Mantiz Sign Strategic Distribution Partnership to Expand Spine Implant Portfolio
Cytogen, a precision medical bio company, and Mantiz, a spinal implant manufacturing company, have signed a domestic distribution agreement covering Mantiz’ full spinal implant portfolio. The agreement was signed on the 27th and formalizes a mid- to long-term strategic partnership between the two companies. The contract goes beyond a traditional product-specific distribution … [Read more...] about Cytogen and Mantiz Sign Strategic Distribution Partnership to Expand Spine Implant Portfolio
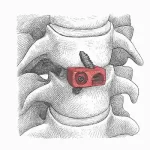
SC MEDICA Announces World’s First 100% Percutaneous, Tubeless Facet Cage Arthrodesis
January 27, 2026 – SC MEDICA today announced a world first: the successful fully percutaneous, tubeless facet cage arthrodesis. SC MEDICA’s FFX® Facet FiXation system was used to treat facetogenic low back pain. Low back pain is the most common pain syndrome and an enormous burden for society. Spinal facet joints are responsible for up to 45% of chronic low back pain cases … [Read more...] about SC MEDICA Announces World’s First 100% Percutaneous, Tubeless Facet Cage Arthrodesis
We are proud to announce that Ruthless Spine will be a Sponsor of SPINEMarketGroup in 2026!
Thank you, Ruthless Spine! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to start this journey together! About Ruthless Spine Ruthless Spine’s mission is to bring “zen” back to spine surgery by addressing the challenges faced by spinal surgeons, such as the need for significant … [Read more...] about We are proud to announce that Ruthless Spine will be a Sponsor of SPINEMarketGroup in 2026!
Medtronic Named in Florida Lawsuit Over Alleged Pedicle Screw Failure
A product liability lawsuit filed in Florida alleges that pedicle screws implanted during a spinal fusion procedure fractured inside a patient’s body, leading to multiple additional surgeries after the spinal hardware reportedly failed. The complaint was initially filed in the Circuit Court for Leon County, Florida on December 17 and was later removed on January 20 to the … [Read more...] about Medtronic Named in Florida Lawsuit Over Alleged Pedicle Screw Failure
Spine Innovation Announces FDA 510(k) Clearance for LOGIC™ Titanium Expandable Interbody System
CORONADO, Calif., Jan. 26, 2026 /PRNewswire/ -- Spine Innovation, LLC, a medical device startup that develops novel interbody fusion implants, announced today that is has received FDA 510(k) clearance to market the LOGIC™ Titanium Implant System. The LOGIC™ Implant System is the next generation LOGIC™ implant incorporating OsteoSync™ Ti, a patented, pure titanium … [Read more...] about Spine Innovation Announces FDA 510(k) Clearance for LOGIC™ Titanium Expandable Interbody System
(UPDATED 2026) More Than 100 Options, No Single Winner: Understanding Cervical Stand-Alone Cages!
The cervical degenerative spine market has changed over the years. At the beginning of the 90s, cervical plates were the gold standard for degenerative indications. Later, in that decade, interbody cages appeared for the first time. They provided primary fixation solving the problem of bone graft resorption. However, they have to be used in conjunction with a cervical … [Read more...] about (UPDATED 2026) More Than 100 Options, No Single Winner: Understanding Cervical Stand-Alone Cages!
We are proud to announce that GS Medical will be a Sponsor of SPINEMarketGroup in 2026!
Thank you, GS Medical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About GS Medical USA A leader in the surgical spine industry, GS Medical is a supplier of spinal implants and instrumentation and a provider of high-quality … [Read more...] about We are proud to announce that GS Medical will be a Sponsor of SPINEMarketGroup in 2026!
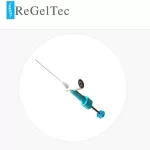
Pain Physician Study Shows Percutaneous Hydrogel Implant for Chronic Low Back Pain Improves Patients’ Pain and Function
BALTIMORE, January 22, 2025 – ReGelTec Inc., a company developing a percutaneous hydrogel implant for the treatment of chronic low back pain caused by degenerative disc disease, announced the publication of a journal article in Pain Physician demonstrating that its HYDRAFIL System for disc augmentation safely generated lasting clinically significant … [Read more...] about Pain Physician Study Shows Percutaneous Hydrogel Implant for Chronic Low Back Pain Improves Patients’ Pain and Function
Curiteva Surpasses More Than 10,000 Levels Successfully Treated
HUNTSVILLE, Ala., January 21, 2026 (Newswire.com) - Curiteva, Inc., a privately held, technology and manufacturing company, is proud to announce the significant achievement of over 10,000 levels successfully treated using the Inspire implant portfolio. With 5,000 interbodies implanted in just the last several months, this milestone underscores the rapid market adoption and … [Read more...] about Curiteva Surpasses More Than 10,000 Levels Successfully Treated
SMAIO Reports 2025 Sales of €9.2m, Representing an Increase of +67%
DALLAS & LYON, France--(BUSINESS WIRE)--Regulatory News: SMAIO (Software, Machines and Adaptive Implants in Orthopaedics – Euronext Growth Paris, ISIN: FR0014005I80 / Ticker: ALSMA, eligible for the PEA-PME scheme), a French-American player specialized in complex spine surgery, offering an integrated pre, intra, and post-operative solution based … [Read more...] about SMAIO Reports 2025 Sales of €9.2m, Representing an Increase of +67%
Medtronic Korea Launches Kanghui, a New Value-Focused Spine Portfolio
Medtronic Korea, the Korean subsidiary of global healthcare technology company Medtronic, has introduced Kanghui, a new spine product portfolio aimed at expanding access to high-quality spinal care at a more accessible cost. Kanghui has been developed to bring Medtronic’s technological expertise and rigorous quality management systems to spinal procedures across the … [Read more...] about Medtronic Korea Launches Kanghui, a New Value-Focused Spine Portfolio
Expanding Innovations Caps Record Year with Breakout Q4 Growth
MOUNTAIN VIEW, Calif., Jan. 21, 2026 /PRNewswire/ -- Expanding Innovations™ (EI) today announced record sales and revenue performance for the fourth quarter of 2025. The company reported 45% annual growth compared to December 2024 and 41% year-over-year quarterly growth for Q4 over Q4'24. This outstanding annual growth marked the largest annual revenue increase in … [Read more...] about Expanding Innovations Caps Record Year with Breakout Q4 Growth
Omnia Medical Announces Commercial Launch of FDA-Cleared PsiF DNA™ System
MORGANTOWN, W.Va., Jan. 20, 2026 /PRNewswire/ -- Omnia Medical, a medical technology company developing surgical solutions for spine and interventional pain physicians, today announced the commercial launch of its FDA-cleared PsiF DNA™ Sacroiliac Joint Stabilization System. In May 2025, Omnia Medical received U.S. Food and Drug … [Read more...] about Omnia Medical Announces Commercial Launch of FDA-Cleared PsiF DNA™ System